- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Ali Jalali, MD, PhD, Department of Neurosurgery, Baylor College of Medicine, Houston, United States. .
DOI:10.25259/SNI_255_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prazwal Athukuri, A. Basit Khan, Ron Gadot, Monira Haque, Sungho Lee, K. Kelly Gallagher, Martha P. Mims, Gustavo A Rivero, Andreia Barbieri, Akash J. Patel, Ali Jalali. Myeloid sarcoma of the skull base: A case report and systematic literature review. 27-May-2022;13:220
How to cite this URL: Prazwal Athukuri, A. Basit Khan, Ron Gadot, Monira Haque, Sungho Lee, K. Kelly Gallagher, Martha P. Mims, Gustavo A Rivero, Andreia Barbieri, Akash J. Patel, Ali Jalali. Myeloid sarcoma of the skull base: A case report and systematic literature review. 27-May-2022;13:220. Available from: https://surgicalneurologyint.com/surgicalint-articles/11623/
Abstract
Background: Myeloid sarcoma (MS), or chloroma, is a rare extramedullary malignant tumor that consists of undifferentiated granulocytic cells, and it is most commonly associated with acute myeloid leukemia (AML). Intracranial MS accounts for 0.4% of MS cases, and involvement of the skull base and visual dysfunction is rarely reported. However, the optimal treatment and response to treatment of skull base MS in the presence of visual symptoms is unknown.
Case Description: A 30-year-old male with a history of AML presented with rapidly progressive vision loss and a sellar and parasellar mass with bilateral cavernous sinus and optic nerve encasement. The patient underwent endoscopic endonasal transsphenoidal biopsy revealing intracranial MS. He was treated postoperatively with high-dose intravenous and intrathecal cytarabine and had complete restoration of his vision by postoperative day 11. A systematic review of the literature identified six cases of skull base MS, five of whom presenting with visual symptoms. All patients underwent systemic chemotherapy with cytarabine and/or cyclophosphamide, with infrequent use of intrathecal chemotherapy or radiation. Those with reported visual outcomes were diagnosed 4 months or longer after symptom onset and demonstrated no visual improvement with treatment.
Conclusion: Skull base MS is a rare disease entity with a high prevalence of visual dysfunction. Our patient’s complete disappearance of intracranial disease and resolution of visual symptoms with systemic and intrathecal chemotherapy highlight the importance of timely diagnosis and appropriate treatment without a need for direct surgical decompression.
Keywords: Acute myeloid leukemia, Chloroma, Myeloid sarcoma, Parasellar, Skull base
BACKGROUND
Myeloid sarcomas (MSs) are rare extramedullary manifestations of hematologic malignancies such as acute myeloid leukemia (AML), chronic myeloid leukemia (CML), or other myeloproliferative disorders. Frequently termed chloromas due to their green hue, MS is rare with an incidence of 2.5–9% in AML. Moreover, while the mean age of MS is 34.8 years, 60% of all cases of MS occur in children <15 years of age.[
MS masses typically manifest within bony or periosteal structures due to direct spread from bone marrow. Due to its rarity, MS is a challenging diagnosis to make with an overall misdiagnosis rate as high as 40%.[
IMS is rare, with an estimated incidence of 0.4% in patients with AML and has a mixed prognosis with 84-month overall survival rate of 58.6%.[
The most common locations for IMS are the temporal lobe, cerebellum, and falcine or parasagittal locations, in sum accounting for 30.9% of IMS.[
METHODS
All information pertaining to this case was obtained from the electronic medical record. Patient’s informed consent was not needed since no identifiable information was disclosed. A simultaneous systematic review of the literature using the online PubMed and EMBASE was conducted using the dedicated search terms “sellar” or “skull base” and “MS” or “chloroma.” Given limited results, no date restrictions were set. Articles were included specific case details on skull base MS masses which were reported. Abstracts were screened sequentially for content and relevance; articles were excluded if full text was unavailable in English. The remaining articles were read in full.
CLINICAL PRESENTATION
The patient is a 30-year-old man with a history of AML diagnosed 7 years prior and treated with systemic chemotherapy treatment with fludarabine, idarubicin, and cytarabine. The patient has been in clinical remission for 1 year after diagnosis, but was lost to follow-up. He presented to our service complaining of rapidly progressive bilateral vision loss and diplopia for the past 2 months. Previously, he had presented to an optometrist who prescribed new glasses. He, additionally, reported 2 months of headaches, nausea, vomiting, and a 26-pound weight loss. Imaging revealed a sellar mass consistent with a pituitary lesion but without the typical appearance of a pituitary macroadenoma. Examination of the right eye revealed restricted extraocular movements in all directions, ptosis, a relative afferent pupillary defect, and minimal light perception. The left eye demonstrated normal extraocular movements and normal pupillary reactivity, but severely restricted visual fields and decreased acuity of foveal vision. Notably, the patient had rapid fluctuations in his visual examination. On dilated fundoscopic examination, there was bilateral Grade IV papilledema.
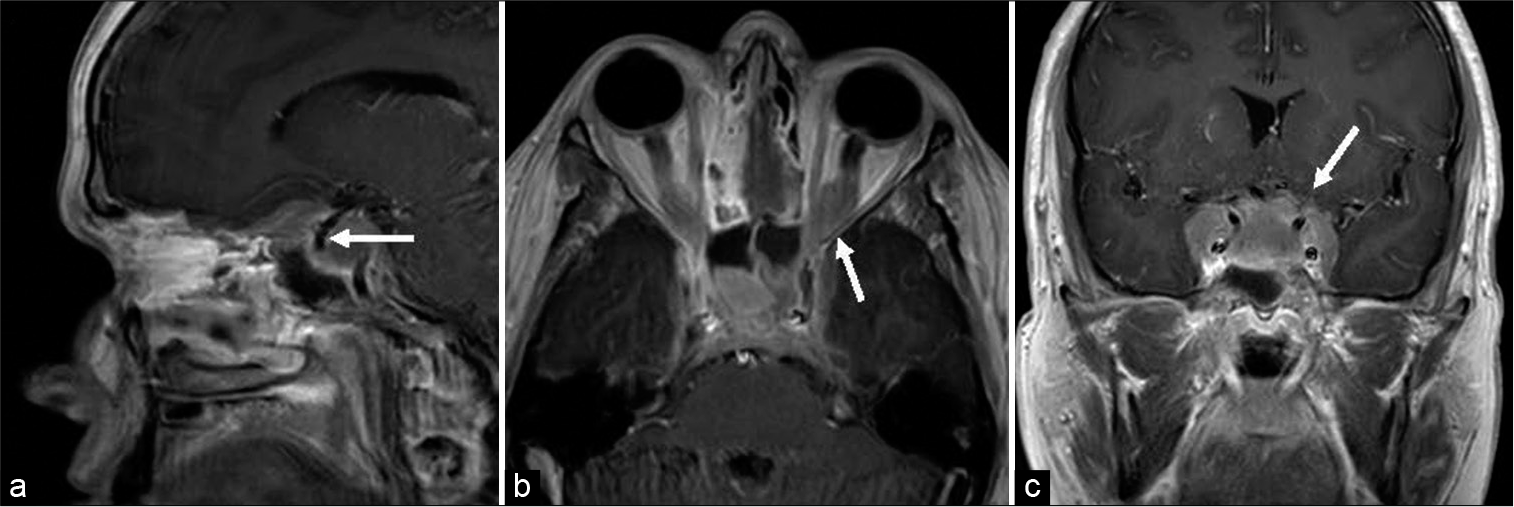
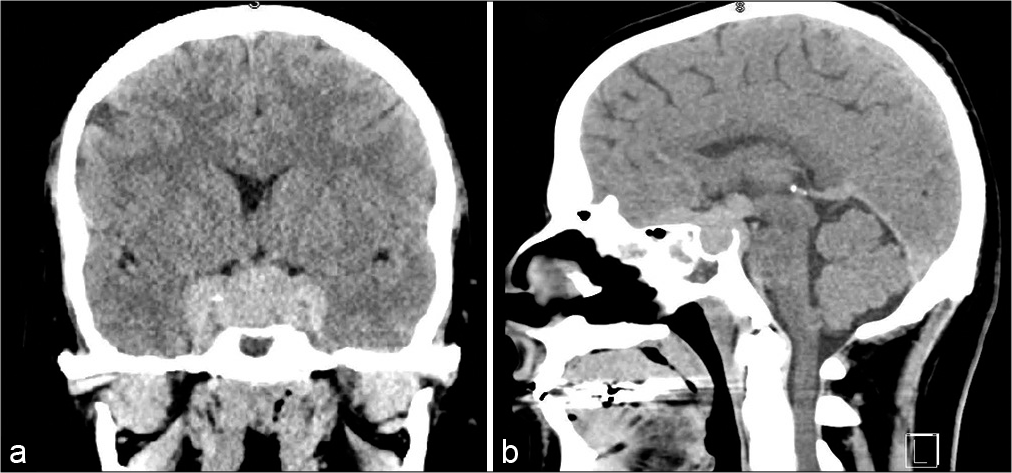
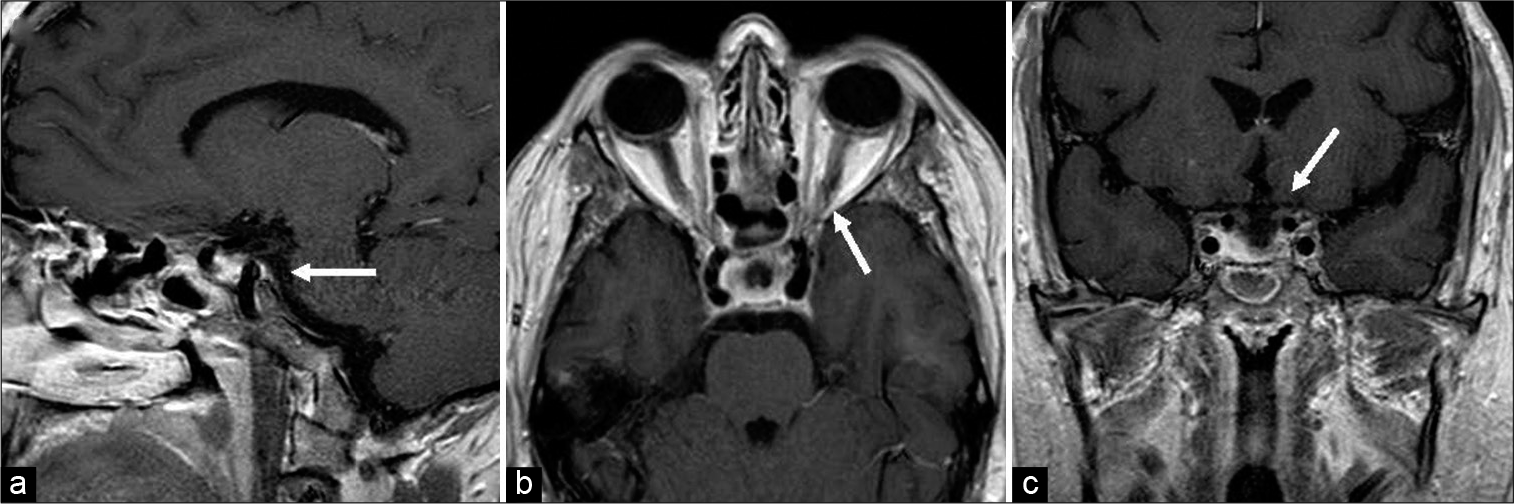
Magnetic resonance imaging (MRI) demonstrated a lobulated extra-axial mass centered in the sella turcica and expanding in multiple directions along the floor of the anterior cranial fossa and crossing bilateral cavernous sinuses into the middle cranial fossae. A component of the mass additionally extended posteriorly and superiorly into the suprasellar cistern and well as inferiorly in the prepontine cistern. Extracranially, the mass was noted to extend into bilateral orbital apices as well as the sphenoid and ethmoid sinuses. Bilateral internal carotid arteries and cavernous sinus contents as well as optic nerves were encased within the tumor. The mass was hypointense on T1 and isointense on T2 with homogenous enhancement on postcontrast T1 [
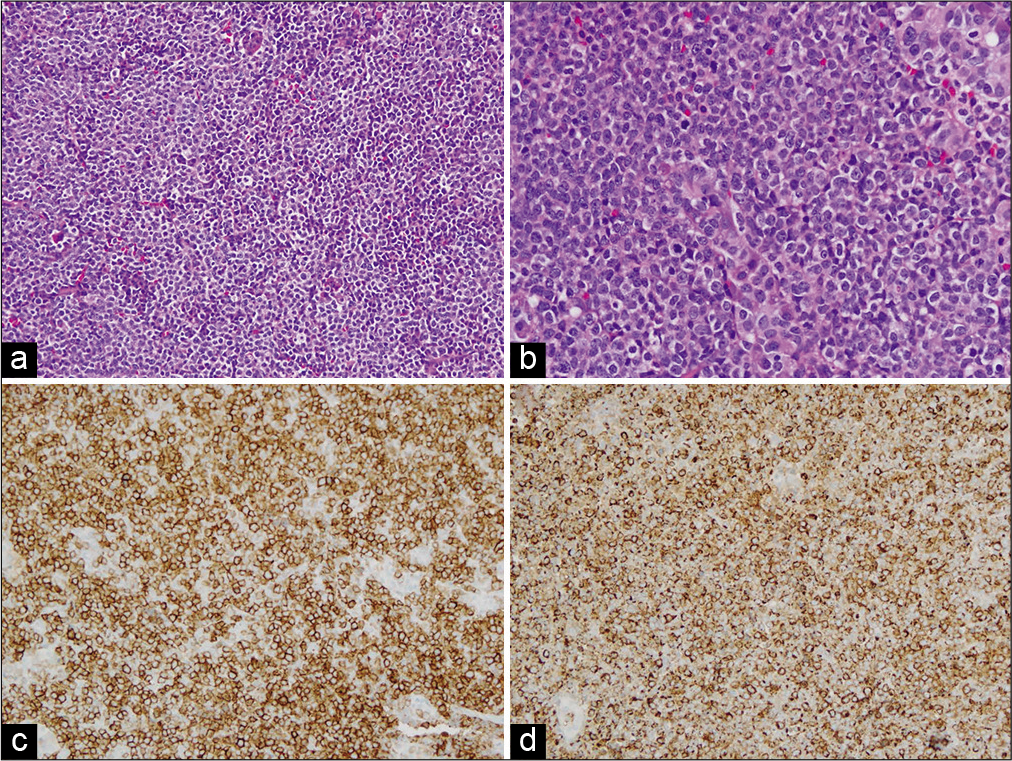
An endoscopic endonasal transsphenoidal biopsy was performed. The intrasphenoidal contents consisted of mucosal cysts with mostly necrotic debris and some viable tissue, which was removed and sent for examination. The anterior wall of the sella was then opened and the mass was encountered immediately under the dura. This was debulked and sent for frozen and permanent pathology. There was no CSF leak, and a multilayer closure was performed. Pathologic review of the tissue showed sheets of blast cells with immunophenotype consistent with MS [
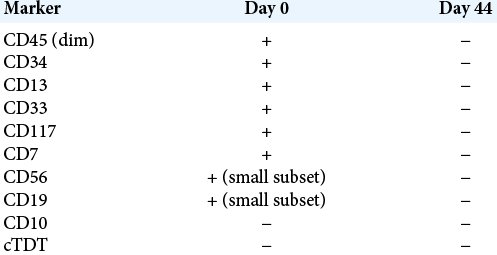
Immediately after surgery, the patient reported only a slight improvement in vision. On postoperative day (POD) 2, high-dose intravenous cytarabine at 2000 mg/m2 over five doses was initiated. On POD 4, his vision had improved to right eye 20/100 and left eye 20/100 centrally when reading at a distance of 12 inches and with normal extraocular movements. By POD 11, the patient had recovered to essentially normal eye function on ophthalmology examination. He was given intrathecal cytarabine on POD 14 over four doses. Abnormal myeloblast ratio in the CSF dropped from 84% on POD 0 to <1% by POD 42 [
SYSTEMATIC REVIEW OF THE LITERATURE
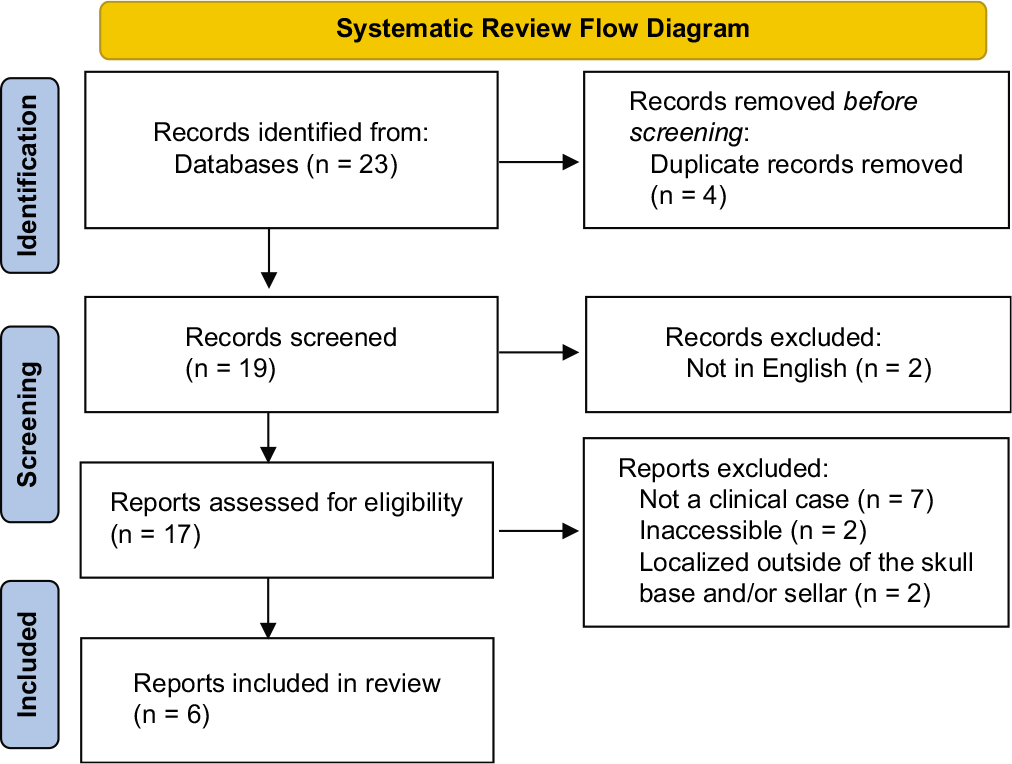
Using dedicated search terms, our systematic review identified 19 articles. After reviewing the abstracts of each, 10 articles were excluded for not satisfying inclusion criteria. The full texts of the remaining nine articles were read in full, after which three additional articles were excluded for lack of specific case details on skull base MS masses. Our systematic review, thus, identified six previously reported cases of skull base MS masses [
Clinical presentation
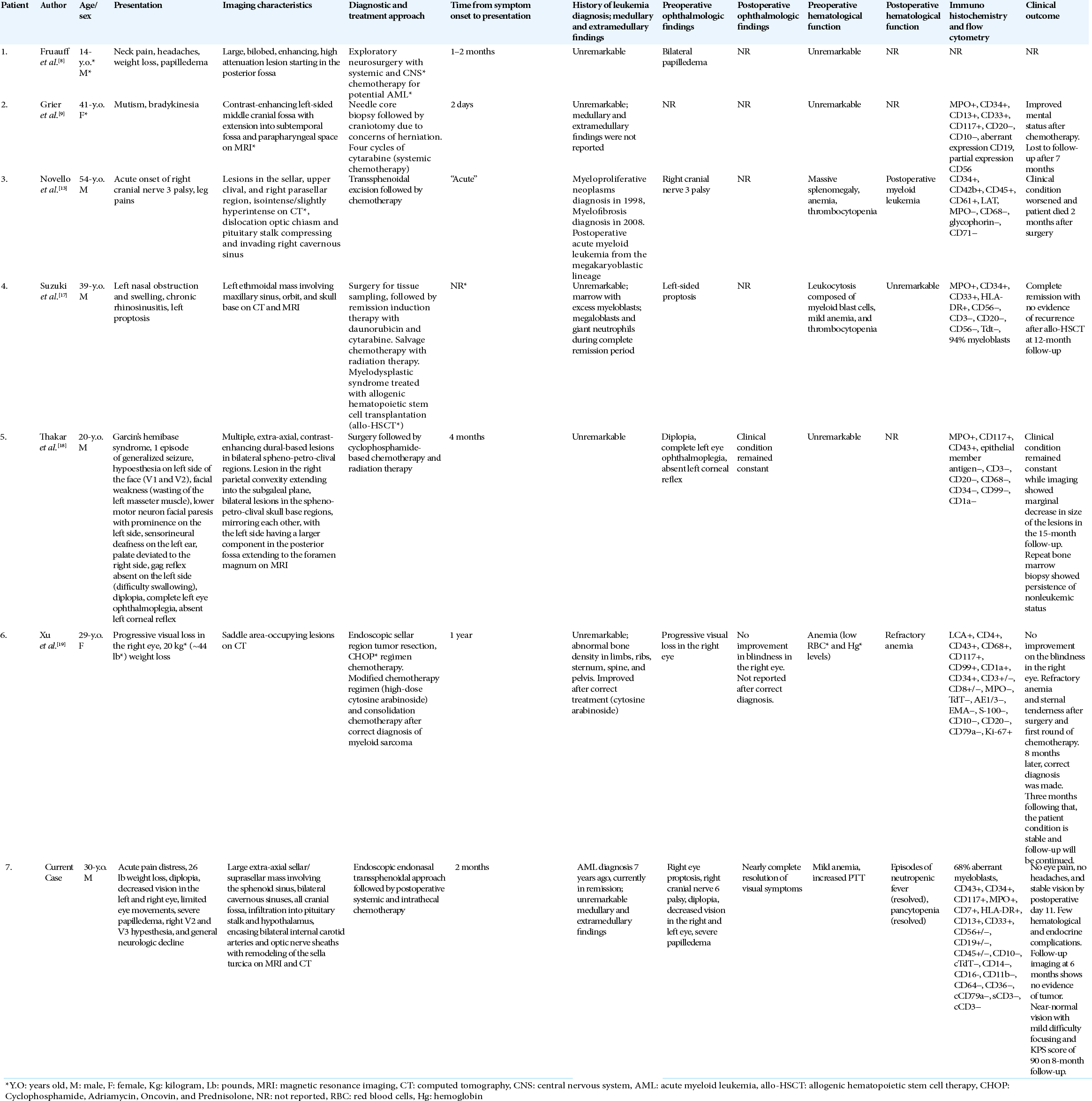
Four patients were male and two patients were female with ages ranging from 14 to 54 years. All patients presented with skull base or sellar region MS masses. Five of the six patients presented with symptoms of ophthalmoplegia (2), vision loss (2), and diplopia (1). Three of the six patients presented within 2 months of symptoms. Two of the six patients presented after 4 months of symptoms. One report did not indicate the time from symptom onset to presentation. Two of the six patients also presented with significant weight loss as noted in our patient. Three of the six patients presented with anemia and thrombocytopenia. Only one patient had a previously diagnosed myeloproliferative neoplasm, whereas the other five patients presented with a MS mass without any history of leukemia.
Imaging findings and laboratory workup
Four of the six patients presented with solitary MS masses in the skull base or sellar region whereas the other two also had abnormal findings on bone marrow biopsy. The MS masses were localized in the anterior, middle, and posterior cranial fossae, including the parasellar, spheno-petro-clival, and orbital regions of the skull base. Five of the six case reports described abnormal findings with immunohistochemistry and flow cytometry consistent with MS, with a common finding of aberrant myeloid cell population. Four cases were CD34+, indicating enhanced progenitor activity.[
Treatment regimen and outcomes
Of cases with vision loss, four reports did not indicate whether the vision findings changed with treatment. The cases with vision loss did not undergo a direct decompression of optic nerves in the acute setting. One patient did not respond to chemotherapy and died after 2 months of worsening clinical condition and progressive systemic disease. All patients underwent surgical biopsy followed by systemic chemotherapy. One case specified that the patient underwent intrathecal chemotherapy. Two of the six patients received radiation therapy as part of multimodal treatment. Four of the six patients received either cytarabine or cyclophosphamide, whereas, in two patients, no details of the regimen were reported.[
DISCUSSION
Here, we report a case of a 30-year-old man with a history of AML in remission who presented with rapidly progressive vision loss. MRI and CT revealed a mass in the sellar and parasellar region with encasement of bilateral optic nerves and cavernous sinus components, which prompted surgical biopsy. Pathological review revealed abnormal myeloblasts and immunophenotype consistent with MS. The patient received systemic cytarabine chemotherapy and had complete recovery of vision and eye movement within a week of starting the treatment. A systematic review of the literature was performed to identify characteristics of skull base or sellar region MS. To the best of our knowledge, there are only six other reported cases in the literature. Similarly to our patient, five of the six previously reported patients presented with visual dysfunction. In all reported cases, surgical biopsy followed by systemic chemotherapy was the mainstay of treatment.
IMS masses appear hyperdense on noncontrast CT and demonstrate intense homogenous enhancement on CT.[
Prognosis of MS is worse when associated with relapse of AML, myeloproliferative disorder, or myelodysplastic syndrome.[
CONCLUSION
We report a case of MS of the sellar and parasellar region diagnosed through endonasal biopsy and managed with chemotherapy. We, additionally, reviewed the extant literature reporting six cases that discuss this rare disease entity. Rapid diagnosis and treatment led to complete resolution of visual and neurological symptoms in our patient without a need for direct surgical decompression. Accordingly, MS should be considered on the differential diagnosis in patients with similar clinical and imaging profiles.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY FIGURE
Acknowledgment
Prazwal Athukuri: data collection, data analysis, and manuscript writing/editing. A. Basit Khan: data collection, data analysis, and manuscript writing/editing. Ron Gadot: data analysis and manuscript writing/editing. Monira Haque: performed all histological/pathological analysis. Sungho Lee: data analysis. K. Kelly Gallagher: data analysis and manuscript writing/editing. Martha P. Mims: data analysis and manuscript writing/editing. Gustavo A. Rivero: data analysis and manuscript writing/editing. Andreia Barbieri: data analysis. Akash J. Patel: data analysis. Ali Jalali: concept ideation, data collection, data analysis, and manuscript writing/editing. All authors read and approved the final manuscript.
References
1. Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH. Myeloid sarcomas: A histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007. 2: 42
2. Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, Perunicic M. Is there a “gold” standard treatment for patients with isolated myeloid sarcoma?. Biomed Pharmacother. 2013. 67: 72-7
3. Avni B, Koren-Michowitz M. Myeloid sarcoma: Current approach and therapeutic options. Ther Adv Hematol. 2011. 2: 309-16
4. Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011. 118: 3785-93
5. Campidelli C, Agostinelli C, Stitson R, Pileri SA. Myeloid sarcoma: Extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009. 132: 426-37
6. Cervantes GM, Cayci Z. Intracranial CNS manifestations of myeloid sarcoma in patients with acute myeloid leukemia: Review of the literature and three case reports from the author’s institution. J Clin Med. 2015. 4: 1102-12
7. Fathi E, Farahzadi R, Sheervalilou R, Sanaat Z, Vietor I. A general view of CD33+ leukemic stem cells and CAR-T cells as interesting targets in acute myeloblatsic leukemia therapy. Blood Res. 2020. 55: 10-6
8. Fruauff AA, Barasch ES, Rosenthal A. Solitary myeloblastoma presenting as acute hydrocephalus: Review of literature, implications for therapy. Pediatr Radiol. 1988. 18: 369-72
9. Grier DD, Al-Quran SZ, Gray B, Li Y, Braylan R. Intracranial myeloid sarcoma. Br J Haematol. 2008. 142: 681
10. Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D. Isolated chloroma: The effect of early antileukemic therapy. Ann Intern Med. 1995. 123: 351-3
11. Khan AB, Goethe EA, Hadley CC, Rouah E, North R, Srinivasan VM. Infundibular epidermoid cyst: Case report and systematic review. World Neurosurg. 2019. 130: 110-4
12. Lee D, Omofoye OA, Nuño MA, Riestenberg RA, Shahlaie K. treatment outcomes of intracranial myeloid sarcomas: A meta-analysis. World Neurosurg. 2021. 148: 29-37
13. Novello M, Coli A, Della Pepa GM, Martini M, Doglietto F, De Stefano V. Myeloid sarcoma with megakaryoblastic differentiation mimicking a sellar tumor. Neuropathology. 2014. 34: 179-84
14. Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M. Myeloid sarcoma: Clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007. 21: 340-50
15. Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014. 32: 1380-9
16. Singh A, Kumar P, Chandrashekhara SH, Kumar A. Unravelling chloroma: Review of imaging findings. Br J Radiol. 2017. 90: 20160710
17. Suzuki J, Harazaki Y, Morita S, Kaga Y, Nomura K, Sugawara M. Myeloid sarcoma of the paranasal sinuses in a patient with acute myeloid leukemia. Tohoku J Exp Med. 2018. 246: 141-6
18. Thakar S, Dadlani R, Ghosal N, Jethwani D, Mahadevan A, Hegde AS. Multiple intracranial de-novo chloromas presenting with Garcin’s syndrome. Clin Neuropathol. 2012. 31: 369-73
19. Xu G, Zhang H, Nong W, Li C, Meng L, Liu C. isolated intracranial myeloid sarcoma mimicking malignant lymphoma: A diagnostic challenge and literature reviews. Onco Targets Ther. 2020. 13: 6085-92
20. Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: A systematic review. Am J Blood Res. 2013. 3: 265-70