- Burdenko Neurosurgical Center, Moscow, Russia.
DOI:10.25259/SNI_180_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anton Konovalov, Dmitry Okishev, Oleg Shekhtman, Yuri Pilipenko, Shalva Eliava. Neuronavigation device for stereotaxic external ventricular drainage insertion. 07-Jun-2021;12:266
How to cite this URL: Anton Konovalov, Dmitry Okishev, Oleg Shekhtman, Yuri Pilipenko, Shalva Eliava. Neuronavigation device for stereotaxic external ventricular drainage insertion. 07-Jun-2021;12:266. Available from: https://surgicalneurologyint.com/surgicalint-articles/10861/
Abstract
Background: The insertion of an external ventricular drainage (EVD) is one of the most frequently used neurosurgical procedures. It is performed to adjust intracranial hypertension in cases of severe craniocerebral injury, acute posthemorrhagic hydrocephalus, meningitis, and oncological diseases related to impaired circulation of cerebrospinal fluid circulation (CSF).
Methods: In 2020, three patients with subarachnoid aneurysmal hemorrhage underwent insertion of an EVD navigation percutaneous stereotaxic device. Three cases introduced.
Results: In all cases, satisfactory EVD functioning was noted during the surgery and during the early postoperative period. The EVD insertion procedure took an average of 10 min. The EVD insertion route calculations using the software took about 5–15 min. No cases showed any infection, hemorrhagic complications, or EVD dysfunction. According to the control brain computed tomography data, the catheter position was satisfactory and corresponded to the target coordinates in all cases.
Conclusion: The use of the device, with its high accuracy and efficiency, can reduce the incidence of unsatisfactory EVD implantation cases in patients with neurosurgical pathology.
Keywords: Device, External ventricular drainage, External ventricular drainage, Hydrocephalus, Insertion
INTRODUCTION
The insertion of external ventricular drainage (EVD) is one of the most frequently used neurosurgical procedures. It is performed to adjust intracranial hypertension in cases of severe craniocerebral injury, acute posthemorrhagic hydrocephalus, meningitis, and oncological diseases related to impaired circulation of cerebrospinal fluid circulation (CSF). As a rule, the insertion of EVD is performed by freehand technique while determining the insertion route of the catheter according to the so-called craniometric points. However, this inevitably leads to the errors, which can range from 12.5% to 40% in some cases.[
MATERIALS AND METHODS
In 2020, three patients with subarachnoid aneurysmal hemorrhage underwent insertion of an EVD navigation percutaneous stereotaxic guide device. All patients underwent a computed tomography (CT) scanning of the brain on admission to the Burdenko National Medical Research Center of Neurosurgery and during the device usage period. A control brain CT was performed on the 1st day after the main microsurgical intervention in relation to the aneurysm. The EVD insertion route’s accuracy, the absence of complications related to the procedure, and the efficiency of the EVD operation were assessed.
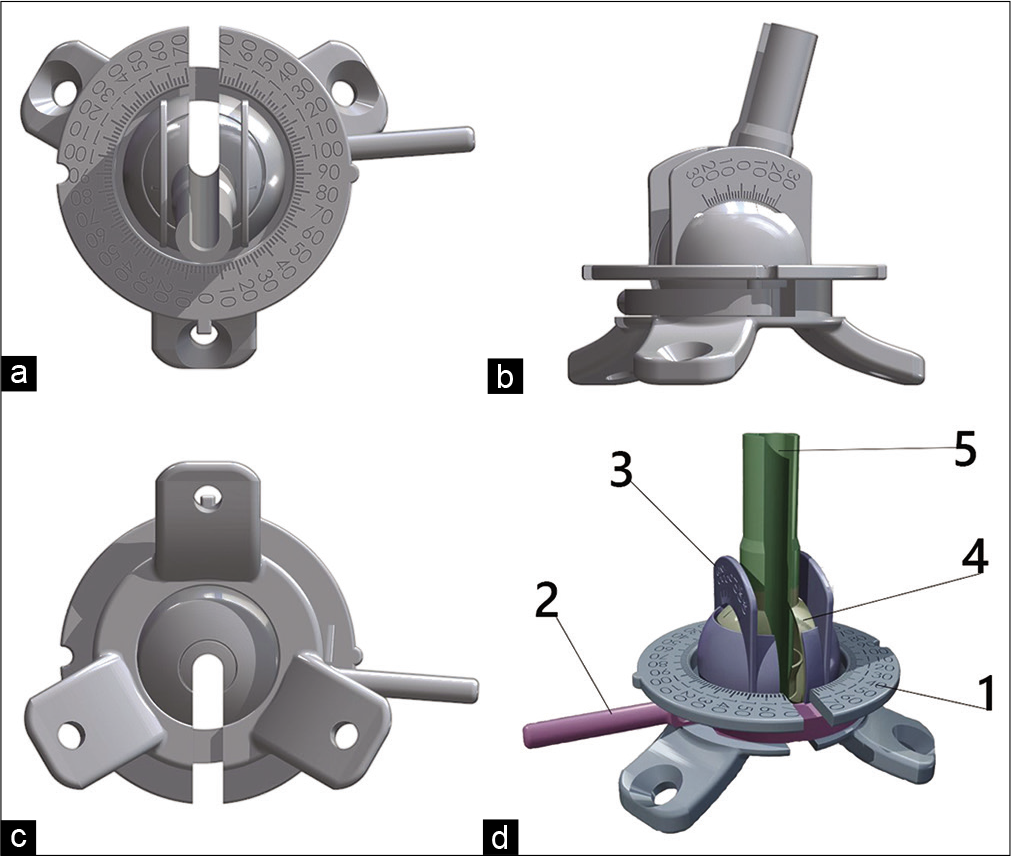
Description of the B-guide device
The guide device contains a retaining ring with three legs, a horizontal scale, a vertical scale, a hinge, and a trocar that fixes the lock [
The horizontal scale is a slotted ring connected to the retaining ring. The vertical scale is a hemisphere with a hole at the top and a slot going down. Two scales in the form of parallel arcs with divisions are installed on the hemisphere. The hinge has a slot and is installed inside the hemisphere of the vertical scale. The trocar also has a slot and is installed inside the hinge. The slots in the retaining ring, horizontal scale, vertical scale, hinge, and trocar are aligned. The device provides the required tool rigidity during the surgery and navigational accuracy, which has been repeatedly confirmed during experiments on phantom models.
It is an experimental device. The model was designed and manufactured by neurosurgical team from Burdenko Neurosurgical Center (Moscow, Russia) and 3D printed from titanium. The national innovative grant was obtained for developing this device.
Use of the B-guide
After selecting the area for the EVD insertion (the right premotor area was used in all cases), the area for the device fixture was prepared by shaving and establishing aseptic conditions. Using three self-tapping titanium Grade 5 bolts, the device’s ring base was fixed through the skin to the cortical plate [
The patient was transported to the CT scanner in the intensive care unit. The brain CT scanning was performed with the device inserted, and then, the patient was transported to the neurosurgical operating room to perform EVD implantation and the main microsurgical intervention, aneurysm clipping. The EVD insertion route was predicted using Inobitec software (
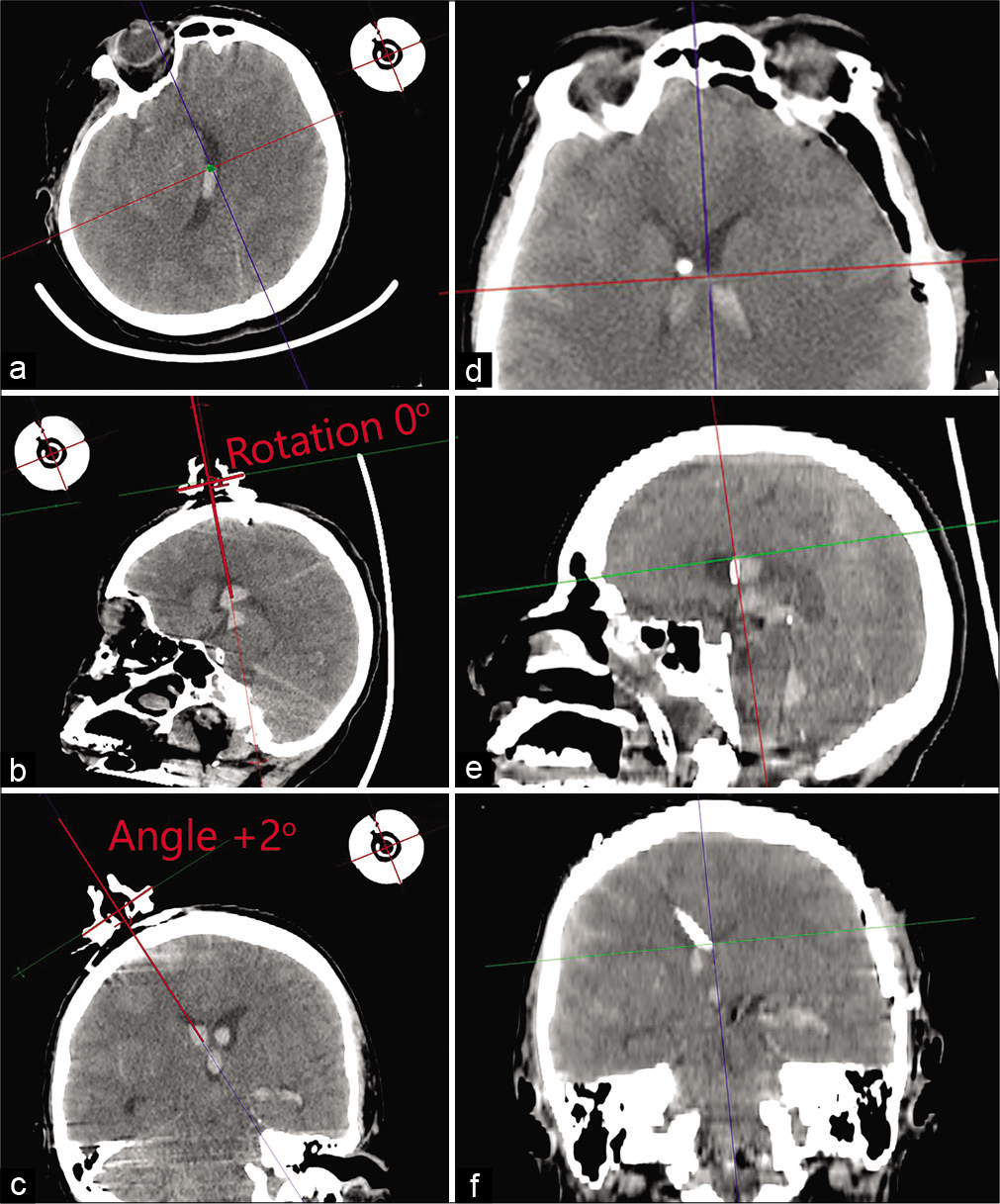
Figure 2:
Clinical example of using a navigation device. Insertion of an external ventricular drainage in the area of the patient’s right lateral ventricle on the 1st day after subarachnoid hemorrhage from an aneurysm of the left internal carotid artery. (a-c) Calculation of parameters for planning a navigation insertion of the external ventricular drainage using the DICOM viewer: the plain of the ring has chosen, the rotation angle and inclination angle calculated with Cobb’s angle tool. Angle of inclination: 2? Angle of rotation: 0?. (d-f) Brain computed tomography after surgery.
The film covering is removed, and additional aseptic processing is performed on the area of the retaining ring and the patient’s soft tissues in the operating room under sterile conditions. A complete assembly of all device elements was carried out. A 5 mm skin incision was made, the previously calculated parameters were applied on the device, and its final fixation was performed. A trephine opening was made with a strictly specified trajectory through the guiding trocar set according to the calculated parameters using a medical drill. The drill diameter was 4 mm, and the drill length was set by considering the trocar length and thickness of the soft tissues and skull bones [
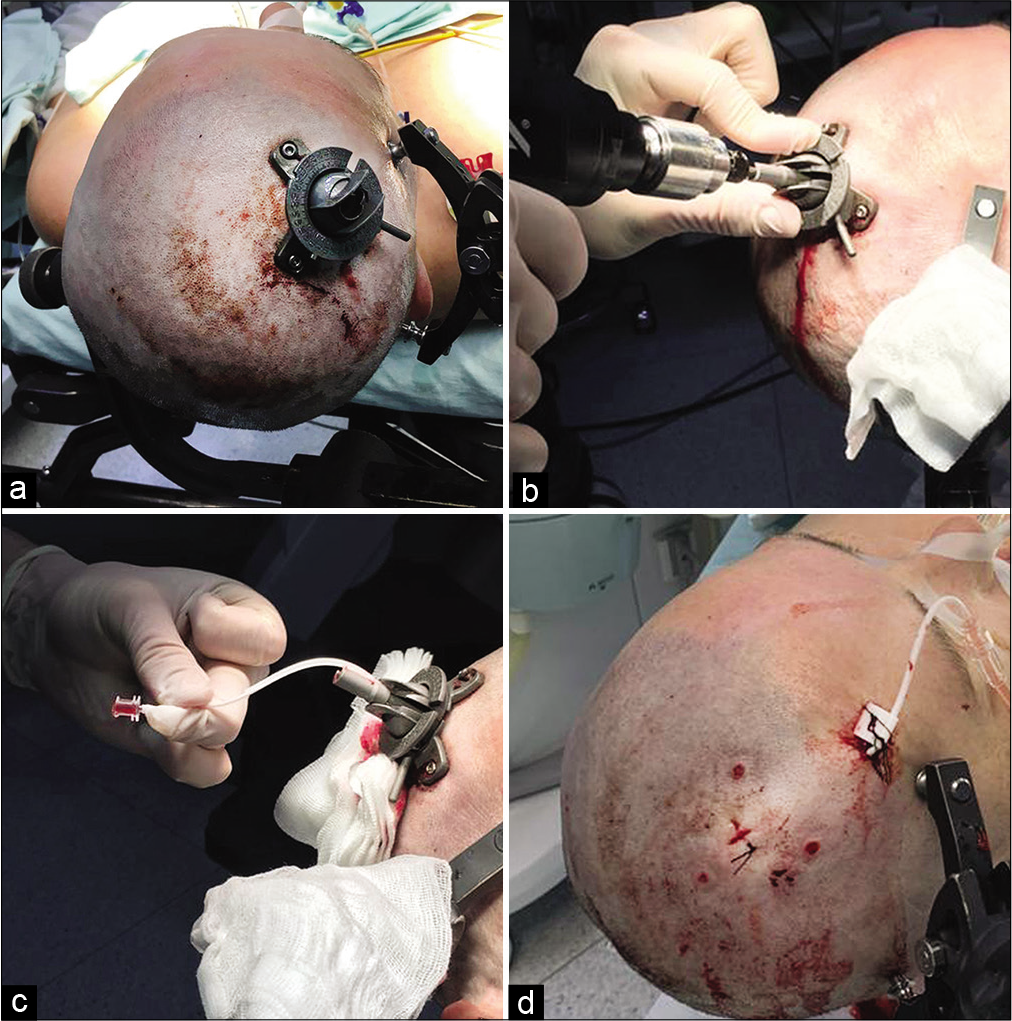
Figure 3:
Clinical example of using a navigation device. Insertion of an external ventricular drainage in the area of the patient’s right lateral ventricle on the 1st day after subarachnoid hemorrhage from an aneurysm of the left internal carotid artery. (a) View of the device after fixation. (b) Device insertion, performing of the stereotaxically specified trephination. (c) Insertion of an external ventricular drainage. (d) Drainage through the contraincision, view of wound after the procedure.
After feeling a slight dip during the drilling procedure, the drill was removed, and the EVD was inserted to a depth of 60 (±1.3) mm from the surface of the patient’s skin. In all cases, the EVD implantation was successful in the first attempt, and xanchromatic CSF was obtained under high pressure. At the stage of tunneling, fixation, and suturing of soft tissues, the drainage was blocked. The device was removed from the skin surface by unscrewing the screws, and the drain tube was taken out of the device through the slots in all the elements of the device. Using a stylet, a contraincision was made through the incision, the EVD was fixed to the skin with a single interrupted suture, and the incision area was covered with a single suture. The EVD was connected to a closed system for passive drainage.
RESULTS
In all cases, satisfactory EVD functioning was noted during the surgery and during the early postoperative period. The EVD insertion procedure took an average of 10 min. The EVD insertion route calculations using the software took about 5–15 min. No cases showed any infection, hemorrhagic complications, or EVD dysfunction. According to the control brain CT data, the catheter position was satisfactory and corresponded to the target coordinates in all cases. Detailed results are given in [
DISCUSSION
The problem of EVD insertion accuracy and methods for achieving the set goals have been studied for a long time.[
The advantages of the device proposed in this study are the provision of stereotaxic accuracy of the EVD insertion, which makes it possible to reduce the risk of an unsatisfactory catheter position, as well as the traumatization of the adjacent anatomical structures during the repeated EVD insertions “by hand.” Notably, the use of the device does not increase the risk of postoperative hemorrhagic or infectious complications. Further study is required for the use of such a navigation device for other neurosurgical diseases, such as a tumor cyst puncture, intracerebral hematomas to perform fibrinolysis, and placement of electrodes for deep brain stimulation or EEG.
CONCLUSION
The proposed device for stereotaxic percutaneous EVD insertion is safe, easy to use, and can be introduced into everyday neurosurgical practice. The use of the B-guide device, with its high accuracy and efficiency, can reduce the incidence of unsatisfactory EVD implantation cases in patients with neurosurgical pathology.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999. 22: 67-93
2. Brenke C, Fürst J, Katsigiannis S, Carolus AE. High accuracy of external ventricular drainage placement using anatomical landmarks. Neurochirurgie. 2020. 66: 435-41
3. Eisenring CV, Burn F, Baumann M, Stieglitz LH, Kockro RA, Beck J. sEVD-smartphone-navigated placement of external ventricular drains. Acta Neurochir (Wien). 2020. 162: 513-21
4. Ghajar JB. A guide for ventricular catheter placement. Technical note. J Neurosurg. 1985. 63: 985-6
5. Ozerov SS, Samarin AE, Mel’nikov AV, Kumirova EV. Placement of a ventricular catheter into narrow lateral ventricles. Popular navigation. Zh Vopr Neirokhir Im N N Burdenko. 2017. 81: 72-6
6. Roach J, Gaastra B, Bulters D, Shtay A. Safety, accuracy, and cost effectiveness of bedside bolt external ventricular drains (EVDs) in comparison with tunneled EVDs inserted in theaters. World Neurosurg. 2019. 125: e473-8
7. Thomale UW, Schaumann A, Stockhammer F, Giese H, Schuster D, Kästner S. GAVCA study: Randomized, multicenter trial to evaluate the quality of ventricular catheter placement with a mobile health assisted guidance technique. Neurosurgery. 2018. 83: 252-62