- Department of Neurosurgery, Kasturba Medical College, Manipal Academy of Higher Education, Udupi, Karnataka, India.
Correspondence Address:
Girish Menon, Department of Neurosurgery, Kasturba Medical College, Manipal Academy of Higher Education, Udupi, Karnataka, India.
DOI:10.25259/SNI_1076_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Deepika Choudhary, Vaishali Mohan, Arla Sai Varsha, Ajay Hegde, Girish Menon. Neurosurgical emergencies during pregnancy – Management dilemmas. 28-Apr-2023;14:151
How to cite this URL: Deepika Choudhary, Vaishali Mohan, Arla Sai Varsha, Ajay Hegde, Girish Menon. Neurosurgical emergencies during pregnancy – Management dilemmas. 28-Apr-2023;14:151. Available from: https://surgicalneurologyint.com/surgicalint-articles/12300/
Abstract
Background: Neurosurgical emergencies in the obstetric setting pose considerable challenges. Decision-making involves deliberations on the gestational age, critical nature of the illness, timing of surgery, maternal positioning during neurosurgery, anesthesiologic strategies, monitoring of the pregnancy during surgery, and the mode of delivery. The present study discusses the management and ethical dilemmas encountered during the management of six obstetric patients with neurosurgical emergencies.
Methods: A retrospective review of all neurosurgical operations performed between January 2016 and December 2022 were included in the study.
Results: This study includes a series of six pregnant women who presented with neurosurgical emergencies, secondary to freshly diagnosed pathologies in the period 2016–2022. The mean maternal age was 31.33 years. Four of the six patients were in the third semester and two were in the second trimester. The underlying etiologies were as follows: spontaneous intracerebral hypertensive hemorrhage (1), obstructive hydrocephalus due to shunt malfunction (1), brain tumor (02), and compressive spinal cord myelopathy due to tumors (02). Three patients who were near term underwent lower cesarean section followed by emergency neurosurgical procedure in the same sitting. Two second trimester patients continued their pregnancy after the emergency neurosurgical operation. In one patient, in whom a brain tumor was diagnosed near term, underwent neurosurgery 1 week after successful cesarean section. All the six mothers and fetus recovered well, ex3cept two patients who have persisting residual deficits.
Conclusion: Treatment of neurosurgical emergencies during pregnancy needs to be customized depending on the clinical condition of the pregnant woman, prognosis of the disease, gestational age and the status of the pregnancy. With careful planning, timely intervention, consultative decision making and it is possible to achieve the ultimate goal – which is to protect and safeguard the mother and preserve and deliver a viable fetus.
Keywords: Brain tumor, Fetus, Intracerebral hemorrhage, Neurosurgery, Obstetrics, Pregnancy
INTRODUCTION
Pregnant women who require emergency neurosurgical intervention pose major challenges in t decision-making and treatment planning.[
MATERIALS AND METHODS
A retrospective review of all pregnant patients who underwent neurosurgical operation during their pregnancy were included in the study. The study period was between January 2016 and December 2022. Patient demographics, obstetric history, neurosurgical intervention and timing of surgery were reviewed from case records. Outcome was assessed on discharge and on follow up in the out patient clinic.
RESULTS
Case 1
A 25-year-old female homemaker (G2P2L1) with 28 weeks of amenorrhea presented with acute onset painless progressive loss of vision in both eyes. On admission, she had no perception of light in her left eye and could barely perceive hand movements in the temporal field in her right eye. An urgent magnetic resonance imaging (MRI) revealed a suprasellar lesion characteristic of a meningioma [
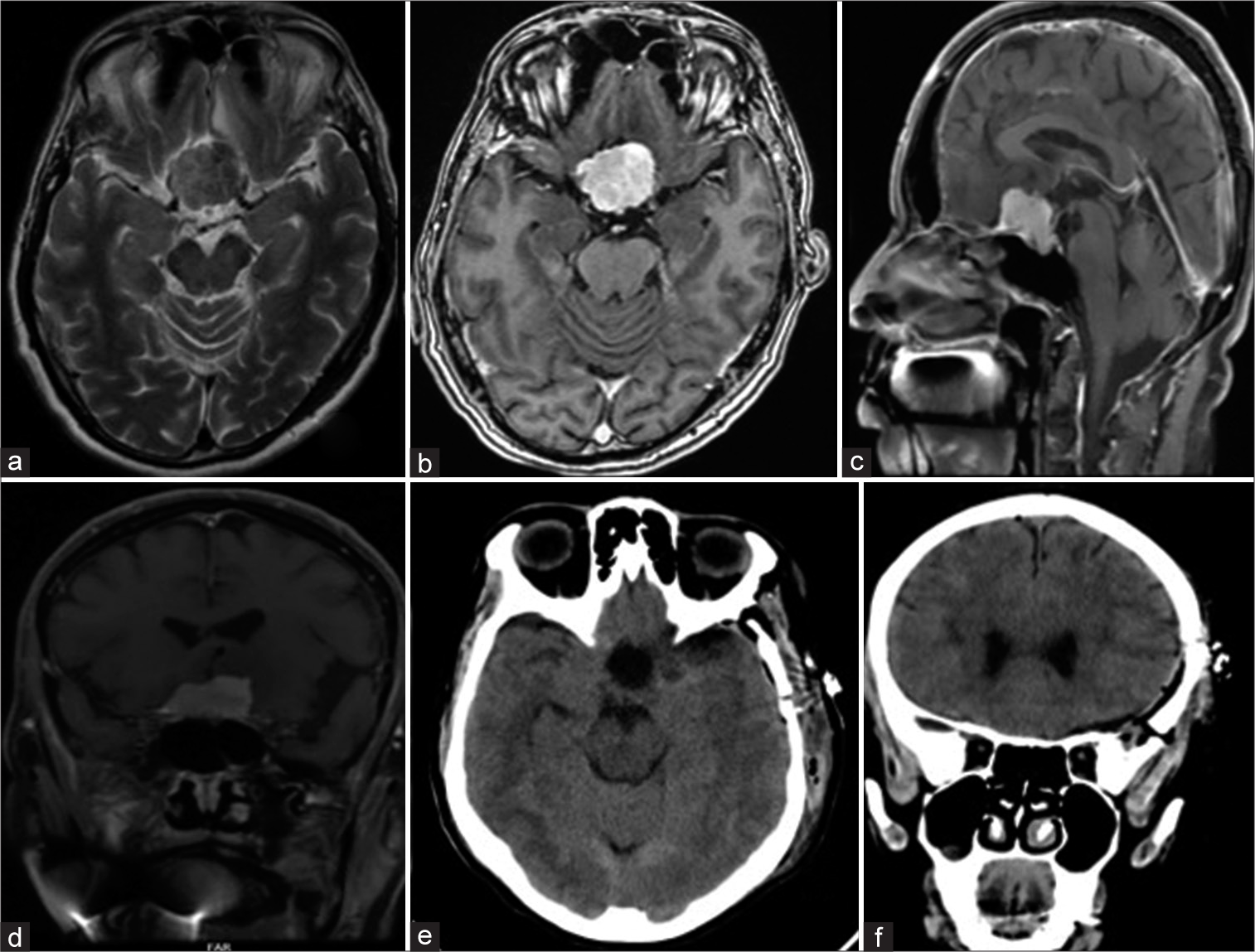
Figure 1:
Magnetic resonance imaging images flair (a), T1 contrast (b), Sagittal T1 (c), and Coronal contrast (d) showing an enhancing suprasellar mass with chiasmal compression. Postoperative axial computerized tomography (CT) scan plain (e) and coronal CT plain (f) showing total excision of the tumor.
Case 2
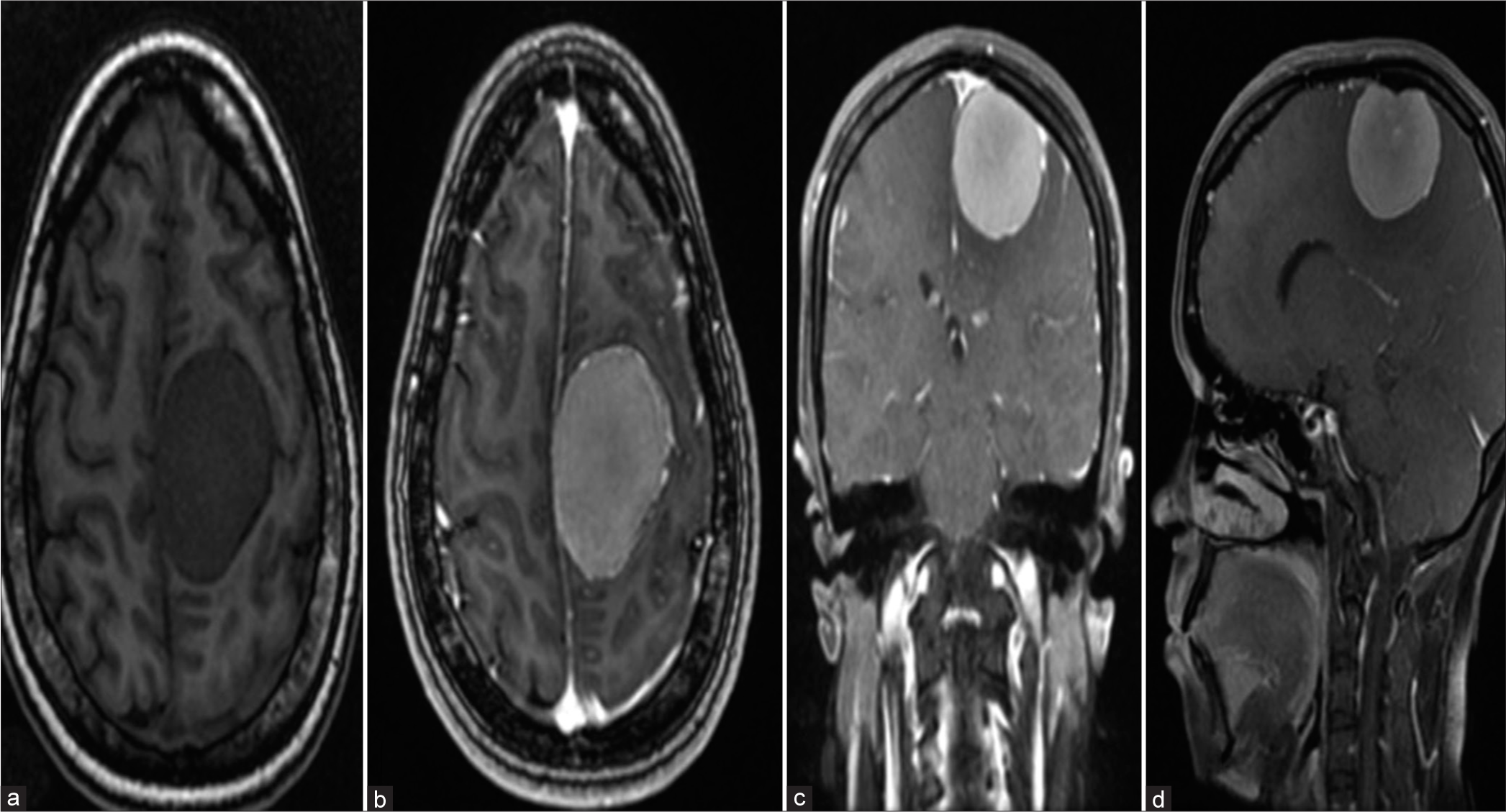
A 35-year-old home maker, (G3 P1 L1 Ab1) presented with one episode of generalized seizure in her 30th week of gestation. On admission, she was neurologically stable and had no deficits. Imaging done revealed a broad based parasagittal mass measuring 5.1 × 4.1 × 3.5 cm with hyperostosis of the adjacent left parietal bone suggestive of a left mid third parasagital meningioma [
Case 3
A 26-year-old female (G1 P1 L1) presented to the neurosurgery department with pain abdomen for 2 days and on and off headache for 3 days at 32+ weeks of gestation. A known diabetic, she had undergone ventriculoperitoneal shunt in childhood for obstructive hydrocephalus due to aqueduct stenosis. As her headache continued to worsen, raised intracranial pressure due to shunt malfunction was suspected which was confirmed on imaging [
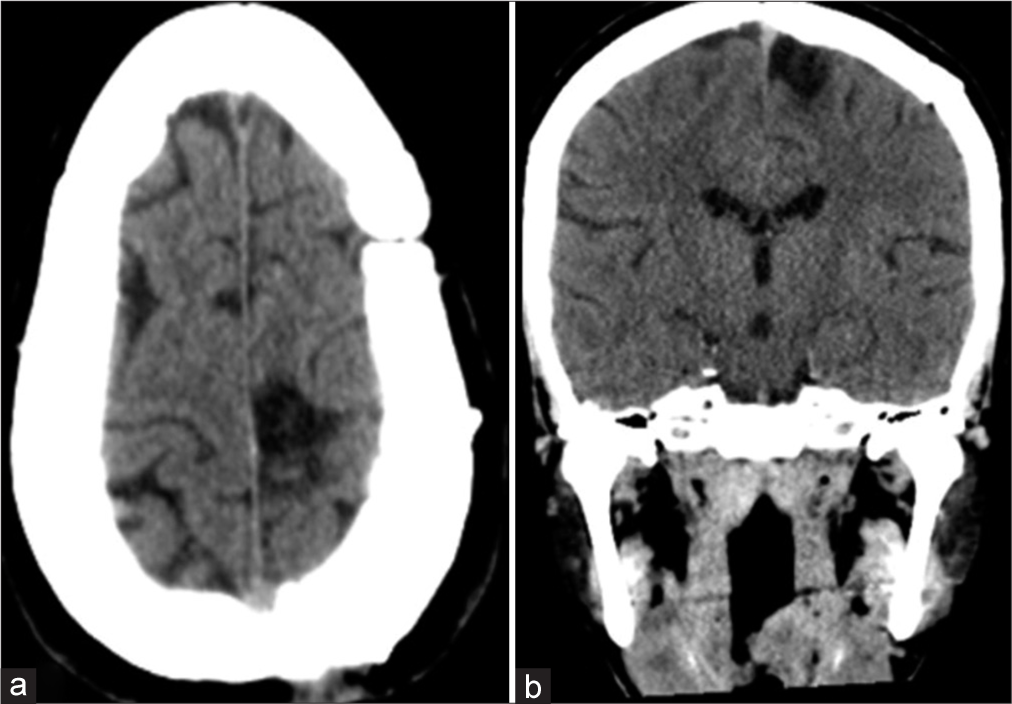
Figure 4:
Computerized tomography (CT) scan axial plain images (a and b) showing ventricular dilatation with periventricular Seepage suggestive of hydrocephalus. Shunt tip can be seen in position in the lateral ventricle. Post endoscopic third ventriculostomy CT scan (c) showing reduction in ventricle size and appearance of sulci and gyri.
Case 4
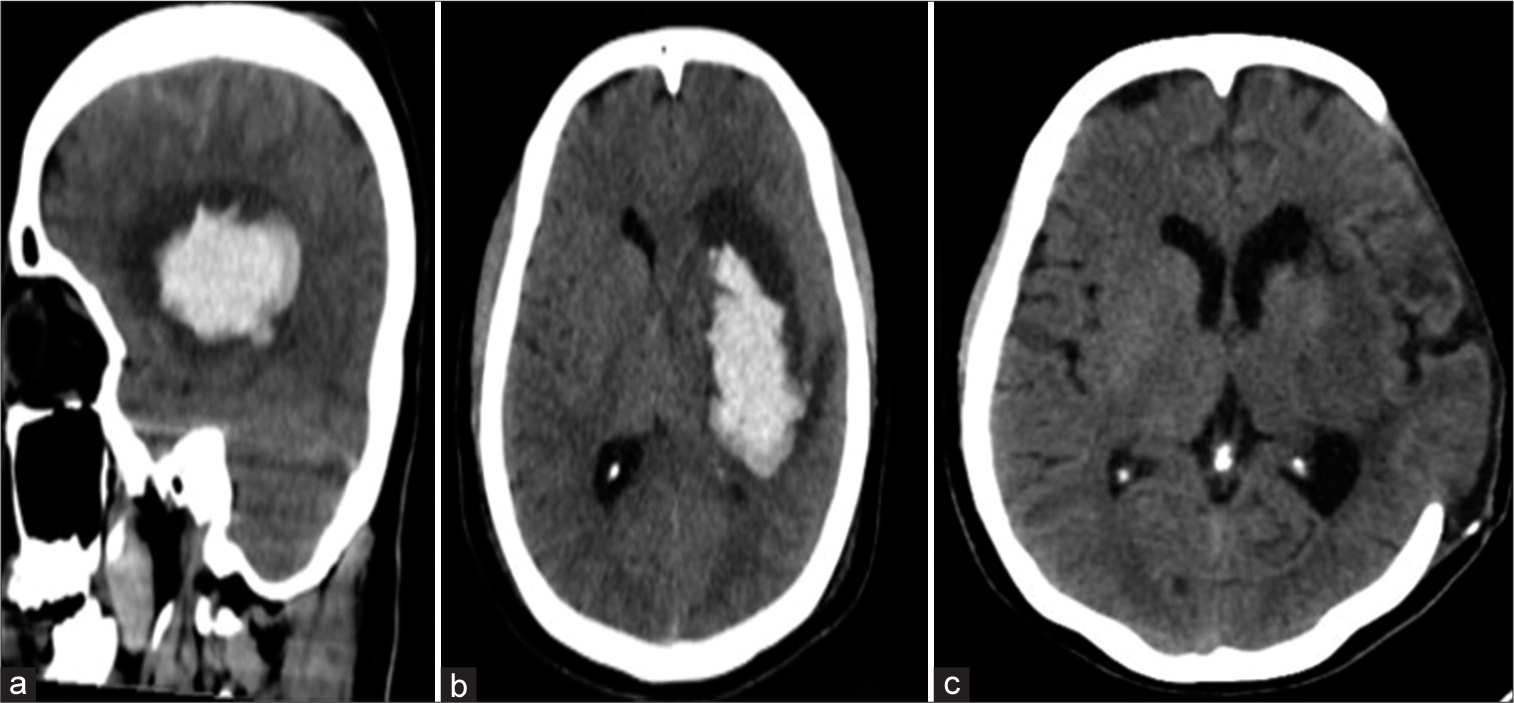
A 36-year old female homemaker (G2 P1 L1) presented to the emergency in her 29th week of gestation with features of accelerated hypertension and impending eclampsia. An emergency lower section cesarean section was done to deliver a preterm baby who was immediately shifted and nursed in the neonatal intensive care unit. Post caesarean section, she developed sudden severe headache, slurring of speech, and right-sided weakness of upper and lower limb and a drop in sensorium. Urgent computerized tomography (CT) scan revealed a large left basal ganglionic hematoma measuring 6.2 × 3.4 × 4.3 with peri-focal edema causing mass effects in the form of compression of ipsilateral lateral ventricle, mild midline shifts [
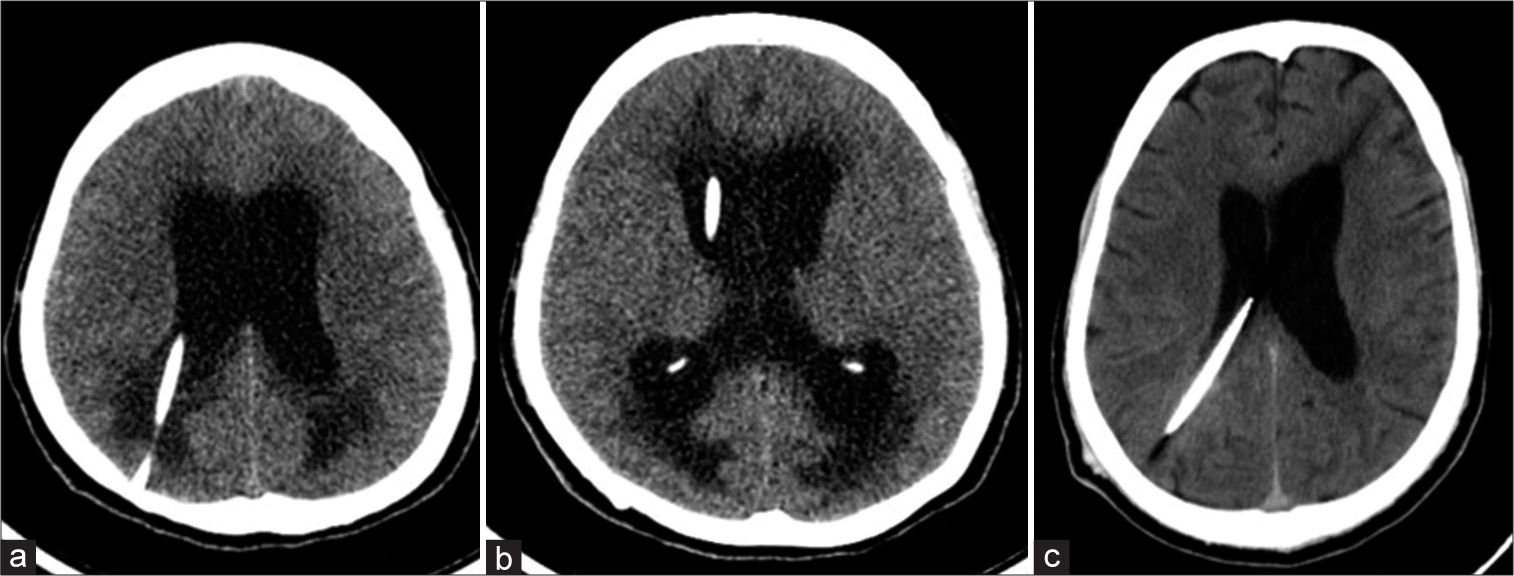
Figure 5:
Computerized tomography scan images plain sagittal (a), axial (b), and coronal (c) showing a large hyperdense Lesion in the left basal ganglionic region suggestive of spontaneous intracerebral hemorrhage. Axial image (c) showing the postoperative scan following decompressive craniectomy and evacuation of the hematoma.
Case 5
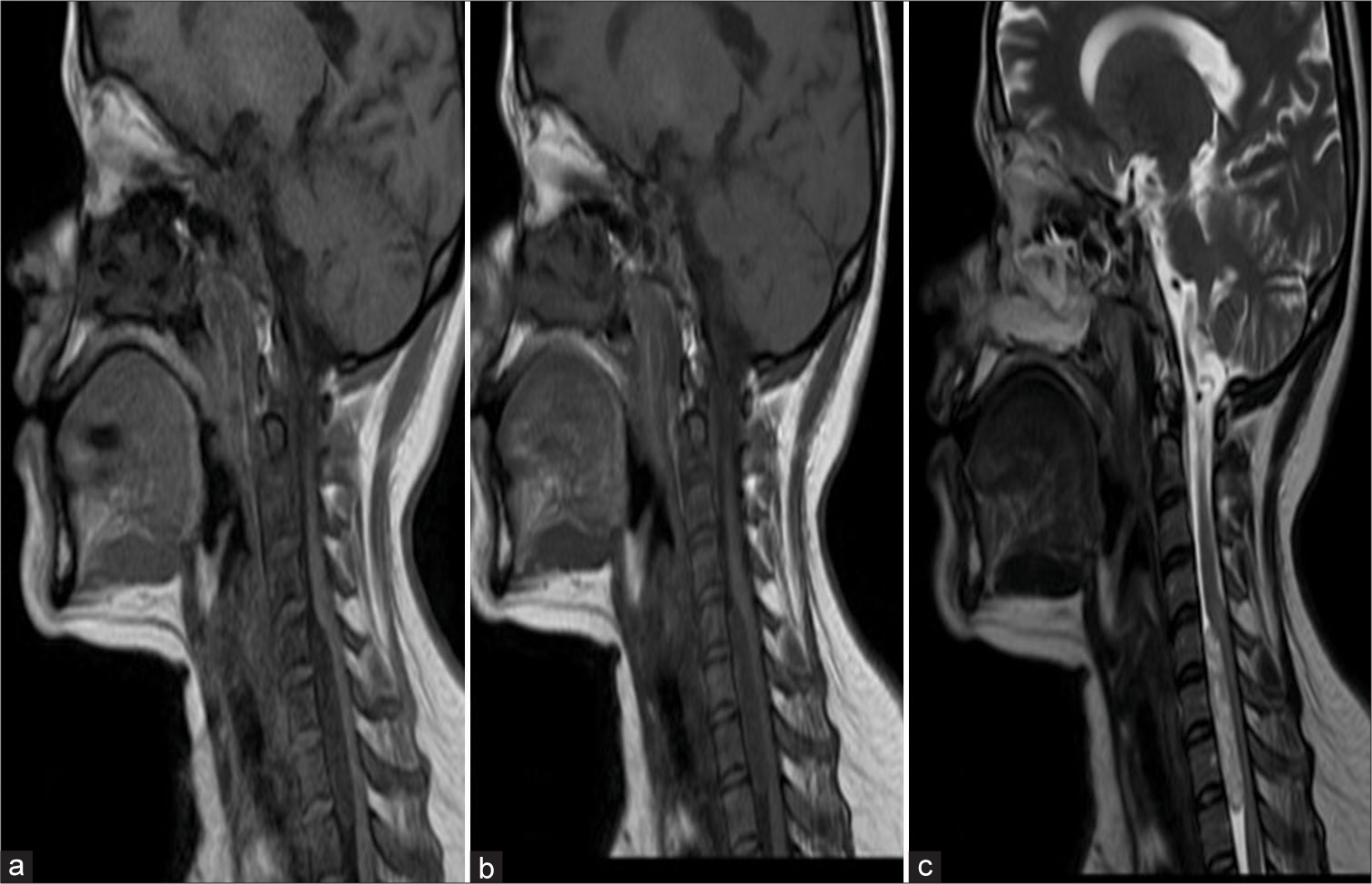
A 33-year-old homemaker (P2L2) presented at 34 + 1 weeks of pregnancy with acute onset, rapidly progressive ascending weakness starting from both lower limbs. On admission, she had Grade 0 power in bilateral lower limbs and Grade 3 power in bilateral upper limbs. She had a sensory level at D1 and had to be catheterized for bladder retention. She also gave history of lower cervical neck pain which had worsened during pregnancy. An urgent MRI of spine revealed a well-defined altered signal intensity intradural extramedullary lesion in the spinal canal from C7 to D1 level, significantly compressing the spinal cord [
Case 6
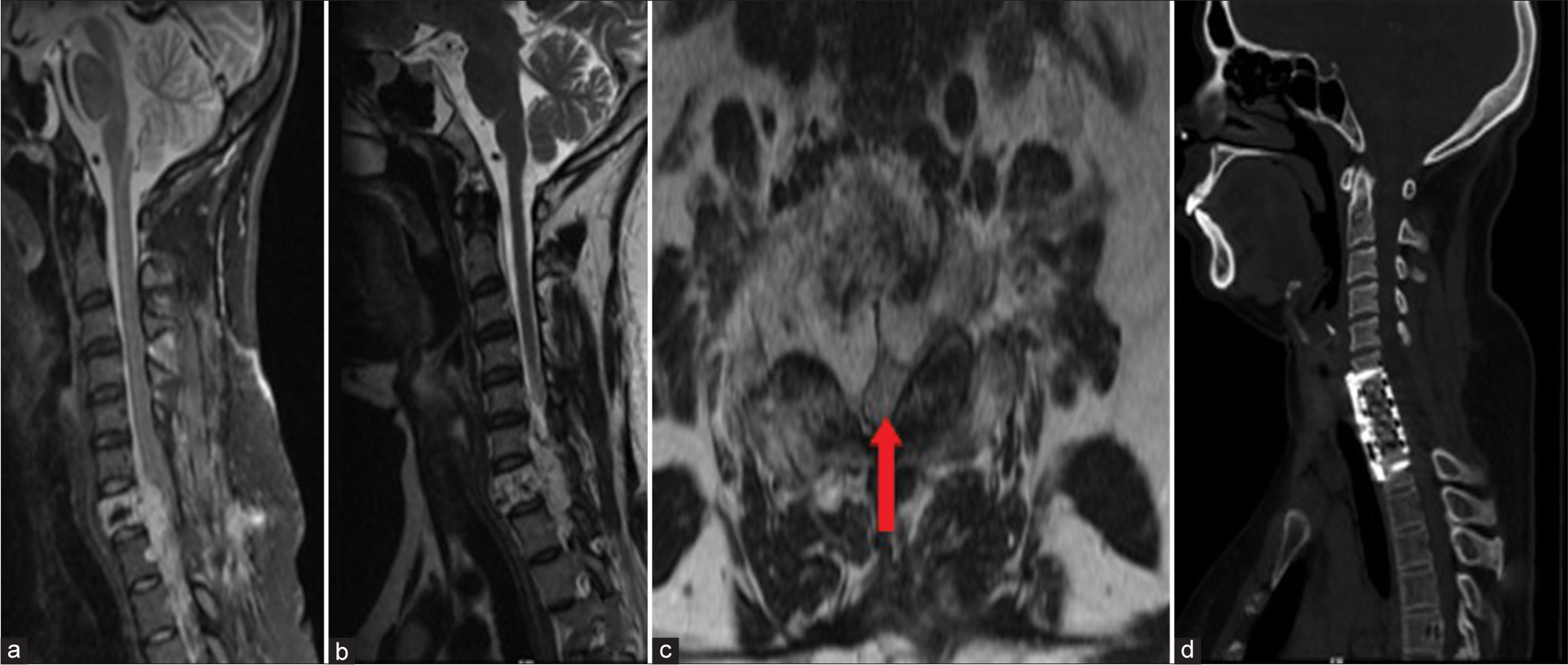
A 33-years-old lady (G2Ab1) presented with progressive weakness of both lower limbs at 5 months of gestation. On admission, she was bedbound with spastic Grade 2–3 power in bilateral lower limbs. MRI revealed a C7 vertebral hemangioma with circumferential cord compression [
Figure 7:
Magnetic resonance imaging images Sagittal T2 (a and b) showing an extradural lesion involving the C7 vertebral body. The cord is seen pushed posteriorly. Axial images (c) show the cord compressed circumferentially anterior by the tumor and posteriorly against the lamina and posterior elements. Red arrow indicates Compressed Spinal Cord (d) Showing postoperative computerized tomography scan following corpectomy with a cage in situ.
DISCUSSION
Pregnant women who require emergency neurosurgical intervention pose major challenges in therapeutic decision-making and treatment planning. Pregnancy is associated with multiple physiological changes which include increased cardiac output, increased intracranial and intra-abdominal pressure, hypercoagulability, and hormonal changes. These changes can promote growth of an existing tumor, induce bleeding in an existing vascular malformation or tumor, or result in a hemorrhagic stroke in a predisposed patient. Such neurosurgical emergencies during pregnancy can result in significant rates of maternal death, fetal loss, miscarriage, and disability in pregnant women.[
Common neurosurgical emergencies during pregnancy
Common neurosurgical emergencies during pregnancy include cereberovenous thrombosis with hemorrhagic transformation, intracranial hemorrhage, hydrocephalus, intracranial and spinal tumors, and head trauma. The incidence of brain tumors in pregnant women ranges between 3.6/million and 3/100,000 while that of vascular lesions in pregnant women is 0.01–0.05%.[
Meningiomas tend to have an increased growth rate during pregnancy and are perhaps the most common of all brain tumors which become symptomatic during pregnancy. They occur with a frequency of 1–4.5/100,000 and are usually detected during the second and third trimesters.[
Vertebral hemangioma is one of the most common benign tumors of the spine seen in approximately 10% of the population.[
The incidence of pregnancy-related intracerebral hemorrhage (ICH) is around 12.2/100,000 deliveries and is responsible for up to 7.1% of all maternal mortality.[
In pregnant women with hydrocephalus, increase of total body water, cardiac output, and central venous pressure, and decrease in plasma osmotic pressure might contribute to development and sudden worsening of symptoms.[
Management - General principle
There are no established guidelines regarding treating pregnant women with neurosurgical emergencies. The treatment plan needs to be customized based on her neurological condition, gestational age, tumor size, and likely pathology. The challenge is in successfully treating the mother’s neurological problem without adversely injuring the growing fetus.[
Timing of surgery
Timing of the surgical intervention is of critical importance as a delay in performing the surgery to safeguard pregnancy may result in irreversible neurological deficits to the mother.[
Case 2 in our series, the patient was diagnosed with a brain tumor late in her pregnancy following an episode of seizure. As seizure was her only symptom and she was neurologically well preserved, it was decided to initiate her on antiseizure medications and craniotomy was performed after completion of normal delivery. This approach, however, they may not be applied in more aggressive or malignant tumors. Case 1 in our series had a suprasellar tumor which suddenly increased in size and caused optic nerve compression with sudden decrease in vision. The pregnancy was in its second trimester. As preserving the mothers vision gained priority over the fetus, craniotomy under general anesthesia was performed explaining the risk for spontaneous termination. A similar situation was encountered in Case 6 for whom we proceeded in two stages. The mother was losing power in her limbs and her precious pregnancy was in early second trimester and the fetus was not viable. Ideal surgical approach involved intraoperative fluoroscopy and positioning in prone position. As a first stage procedure, laminectomy was done in a lateral position to decompress the spinal cord without using any form of intraoperative radiography. Pregnancy was allowed to continue and once the baby was delivered at term complete removal of the tumor and stabilization was done as a second stage procedure.
When the fetus is in the third trimester of gestation and the mother’s neurological illness is critical, an emergency cesarean section should be considered.[
Anesthetic considerations
Anesthetic procedures carry a risk towards the fetus and involve unique challenges.[
Positioning
Positioning the patient for surgery is another concern especially in spinal surgeries.[
Imaging
Diagnostic imaging becomes a challenge during pregnancy. CT scan, the modality of choice for neurosurgical emergencies, carries a risk of radiation exposure to the fetus especially in the first and second trimester. The threshold level for teratogenic risk has been estimated to be >0.05 Gy with the highest risk in the first trimester.[
To summarize, the primary concern in such situations of neurosurgical emergencies complicating pregnancy is the mother and three different options can be adopted.[
CONCLUSION
Neurosurgical emergencies during pregnancy pose considerable ethical and management dilemmas. Along with the underlying ethology, multiple other factors such as gestational period, nature, and severity of the underlying pathology need to be considered before arriving at a decision. With careful planning, timely intervention, consultative decision-making and it is possible to achieve the ultimate goal – which is to protect and safeguard the mother and preserve and deliver a viable fetus.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. ACOG Committee Opinion No. 474: Nonobstetric surgery during pregnancy. Obstet Gynecol. 2011. 117: 420-1
2. Al-Mefty O, Holoubi A, Rifai A, Fox JL. Microsurgical removal of suprasellar meningiomas. Neurosurgery. 1985. 16: 364-72
3. Alvis JS, Hicks RJ. Pregnancy-induced acute neurologic emergencies and neurologic conditions encountered in pregnancy. Semin Ultrasound CT MR. 2012. 33: 46-54
4. Ascanio LC, Maragkos GA, Young BC, Boone MD, Kasper EM. Spontaneous intracranial hemorrhage in pregnancy: A systematic review of the literature. Neurocrit Care. 2019. 30: 5-15
5. Barno A, Freeman DW. Maternal deaths due to spontaneous subarachnoid hemorrhage. Am J Obstet Gynecol. 1976. 125: 384-92
6. Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL. Intracerebral hemorrhage in pregnancy: Frequency, risk factors, and outcome. Neurology. 2006. 67: 424-9
7. Chi JH, Manley GT, Chou D. Pregnancy-related vertebral hemangioma. Case report review of the literature and management algorithm. Neurosurg Focus. 2005. 19: E7
8. Cohen-Gadol AA, Friedman JA, Friedman JD, Tubbs RS, Munis JR, Meyer FB. Neurosurgical management of intracranial lesions in the pregnant patient: A 36-year institutional experience and review of the literature. J Neurosurg. 2009. 111: 1150-7
9. Cushing H, Eisenhardt L. Meningiomas arising from the tuberculum sellae: With the syndrome of primary optic atrophy and bitemporal field defects combined with a normal sella turcica in a middle-aged person. Arch Ophthalmol. 1929. 1: 168-206
10. Dinç H, Esen F, Demirci A, Sari A, Gümele HR. Pituitary dimensions and volume measurements in pregnancy and post partum. MR assessment. Acta Radiol. 1998. 39: 64-9
11. Ebner FH, Bornemann A, Wilhelm H, Ernemann U, Honegger J. Tuberculum sellae meningioma symptomatic during pregnancy: Pathophysiological considerations. Acta Neurochir (Wien). 2008. 150: 189-93
12. Esmaeilzadeh M, Hong B, Polemikos M, Al-Afif S, Hermann EJ, Scheinichen D. Spinal emergency surgery during pregnancy: Contemporary strategies and outcome. World Neurosurg. 2020. 139: e421-7
13. Esmaeilzadeh M, Uksul N, Hong B, von Kaisenberg C, Scheinichen D, Lang JM. Intracranial emergencies during pregnancy requiring urgent neurosurgical treatment. Clin Neurol Neurosurg. 2020. 195: 105905
14. Garcia-Bournissen F, Shrim A, Koren G. Safety of gadolinium during pregnancy. Can Fam Physician. 2006. 52: 309-10
15. Haas JF, Jänisch W, Staneczek W. Newly diagnosed primary intracranial neoplasms in pregnant women: A population-based assessment. J Neurol Neurosurg Psychiatry. 1986. 49: 874-80
16. Han IH. Pregnancy and spinal problems. Curr Opin Obstet Gynecol. 2010. 22: 477-81
17. Hortobágyi T, Bencze J, Murnyák B, Kouhsari MC, Bognár L, Marko-Varga G. Pathophysiology of meningioma growth in pregnancy. Open Med (Wars). 2017. 12: 195-200
18. Kanaan I, Jallu A, Kanaan H. Management strategy for meningioma in pregnancy: A clinical study. Skull Base. 2003. 13: 197-203
19. Kasper EM, Hess PE, Silasi M, Lim KH, Gray J, Reddy H. A pregnant female with a large intracranial mass: Reviewing the evidence to obtain management guidelines for intracranial meningiomas during pregnancy. Surg Neurol Int. 2010. 1: 95
20. Kazemi P, Villar G, Flexman AM. Anesthetic management of neurosurgical procedures during pregnancy: A case series. J Neurosurg Anesthesiol. 2014. 26: 234-40
21. Kerschbaumer J, Freyschlag CF, Stockhammer G, Taucher S, Maier H, Thome C. Hormone-dependent shrinkage of a sphenoid wing meningioma after pregnancy: Case report. J Neurosurg. 2015. 124: 137-40
22. Kiroglu Y, Benek B, Yagci B, Cirak B, Tahta K. Spinal cord compression caused by vertebral hemangioma being symptomatic during pregnancy. Surg Neurol. 2009. 71: 487-92
23. Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW. Pregnancy and the risk of stroke. N Engl J Med. 1996. 335: 768-74
24. Laviv Y, Bayoumi A, Mahadevan A, Young B, Boone M, Kasper EM. Meningiomas in pregnancy: Timing of surgery and clinical outcomes as observed in 104 cases and establishment of a best management strategy. Acta Neurochir (Wien). 2018. 160: 1521-9
25. Laviv Y, Ohla V, Kasper EM. Unique features of pregnancy-related meningiomas: Lessons learned from 148 reported cases and theoretical implications of a prolactin modulated pathogenesis. Neurosurg Rev. 2018. 41: 95-108
26. Low JA, Boston RW, Cervenko FW. Effect of low maternal carbon dioxide tension on placental gas exchange. Am J Obstet Gynecol. 1970. 106: 1032-43
27. Lusis EA, Scheithauer BW, Yachnis AT, Fischer BR, Chicoine MR, Paulus W. Meningiomas in pregnancy: A clinicopathologic study of 17 cases. Neurosurgery. 2012. 71: 951-61
28. Mallari RJ, Thakur JD, Griffiths C, Krauss H, Eisenberg A, Barkhoudarian G. Tuberculum sellae meningiomas in pregnancy: 3 Cases treated in the second trimester and literature review. World Neurosurg. 2020. 143: 268-75
29. Priddy BH, Otto BA, Carrau RL, Prevedello DM. Management of skull base tumors in the obstetric population: A case series. World Neurosurg. 2018. 113: e373-82
30. Rayes M, Konyukhov A, Fayad V, Chaturvedi S, Norris G. Good outcome in HELLP syndrome with lobar cerebral hematomas. Neurocrit Care. 2011. 14: 276-80
31. Robba C, Bacigaluppi S, Bragazzi NL, Bilotta F, Sekhon MS, Bertuetti R. Aneurysmal subarachnoid hemorrhage in pregnancy-case series, review, and pooled data analysis. World Neurosurg. 2016. 88: 383-98
32. Roberts JM, Redman CW. Pre-eclampsia: More than pregnancy-induced hypertension. Lancet. 1993. 341: 1447-51
33. Saitoh Y, Oku Y, Izumoto S, Go J. Rapid growth of a meningioma during pregnancy: Relationship with estrogen and progesterone receptors-case report. Neurol Med Chir (Tokyo). 1989. 29: 440-3
34. Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke. 1995. 26: 930-6
35. Swartz RH, Cayley ML, Foley N, Ladhani NN, Leffert L, Bushnell C. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int J Stroke. 2017. 12: 687-97
36. Toossi S, Moheet AM. Intracerebral hemorrhage in women: A review with special attention to pregnancy and the post-partum period. Neurocrit Care. 2019. 31: 390-8
37. Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. 2016. 60: 234-41
38. van Bogaert LJ, Dhai A. Ethical challenges of treating the critically ill pregnant patient. Best Pract Res Clin Obstet Gynaecol. 2008. 22: 983-96
39. Verheecke M, Halaska MJ, Lok CA, Ottevanger PB, Fruscio R, Dahl-Steffensen K. Primary brain tumours, meningiomas and brain metastases in pregnancy: Report on 27 cases and review of literature. Eur J Cancer. 2014. 50: 1462-71
40. Wang LP, Paech MJ. Neuroanesthesia for the pregnant woman. Anesth Analg. 2008. 107: 193-200
41. Watanabe Y, Oki S, Sumida M, Isobe N, Kanou Y, Takeda M. A case of aqueductal stenosis with acute progressive hydrocephalus during pregnancy. No Shinkei Geka. 2005. 33: 59-63