- Department of Neurosurgery, Neuroscience Center Siloam Hospital, Tangerang, Banten, Indonesia
- Department of Radiology, Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Banten, Indonesia.
Correspondence Address:
Julius July, Department of Neurosurgery, Neuroscience Center Siloam Hospital, Faculty of Medicine, Universitas Pelita Harapan, Jenderal Sudirman Boulevard 20, Tangerang, Banten, Indonesia 15810.
DOI:10.25259/SNI_127_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Patrick Putra Lukito1, Julius July1, Vanessa Angelica Suntoro1, Jeremiah Hilkiah Wijaya1, Audrey Hamdoyo1, Nyoman Aditya Sindunata2, Rusli Muljadi2. Neutrophil-to-lymphocyte ratio predicted cerebral infarction and poor discharge functional outcome in aneurysmal subarachnoid hemorrhage: A propensity score matching analysis. 26-May-2023;14:182
How to cite this URL: Patrick Putra Lukito1, Julius July1, Vanessa Angelica Suntoro1, Jeremiah Hilkiah Wijaya1, Audrey Hamdoyo1, Nyoman Aditya Sindunata2, Rusli Muljadi2. Neutrophil-to-lymphocyte ratio predicted cerebral infarction and poor discharge functional outcome in aneurysmal subarachnoid hemorrhage: A propensity score matching analysis. 26-May-2023;14:182. Available from: https://surgicalneurologyint.com/surgicalint-articles/12337/
Abstract
Background: Neutrophil-lymphocyte-ratio (NLR) and platelet-lymphocyte-ratio (PLR) have emerged as potential biomarkers in predicting the outcomes of aneurysmal subarachnoid hemorrhage (aSAH). Since a study was never conducted on the Southeast Asian and Indonesian population, we designed the present study to evaluate the potential of NLR and PLR in predicting cerebral infarction and functional outcomes and find the optimal cutoff value.
Methods: We retrospectively reviewed patients admitted for aSAH in our hospital between 2017 and 2021. The diagnosis was made using a computed tomography (CT) scan or magnetic resonance imaging and CT angiography. Association between admission NLR and PLR and the outcomes were analyzed using a multivariable regression model. A receiver operating characteristic (ROC) analysis was done to identify the optimal cutoff value. A propensity score matching (PSM) was then carried out to reduce the imbalance between the two groups before comparison.
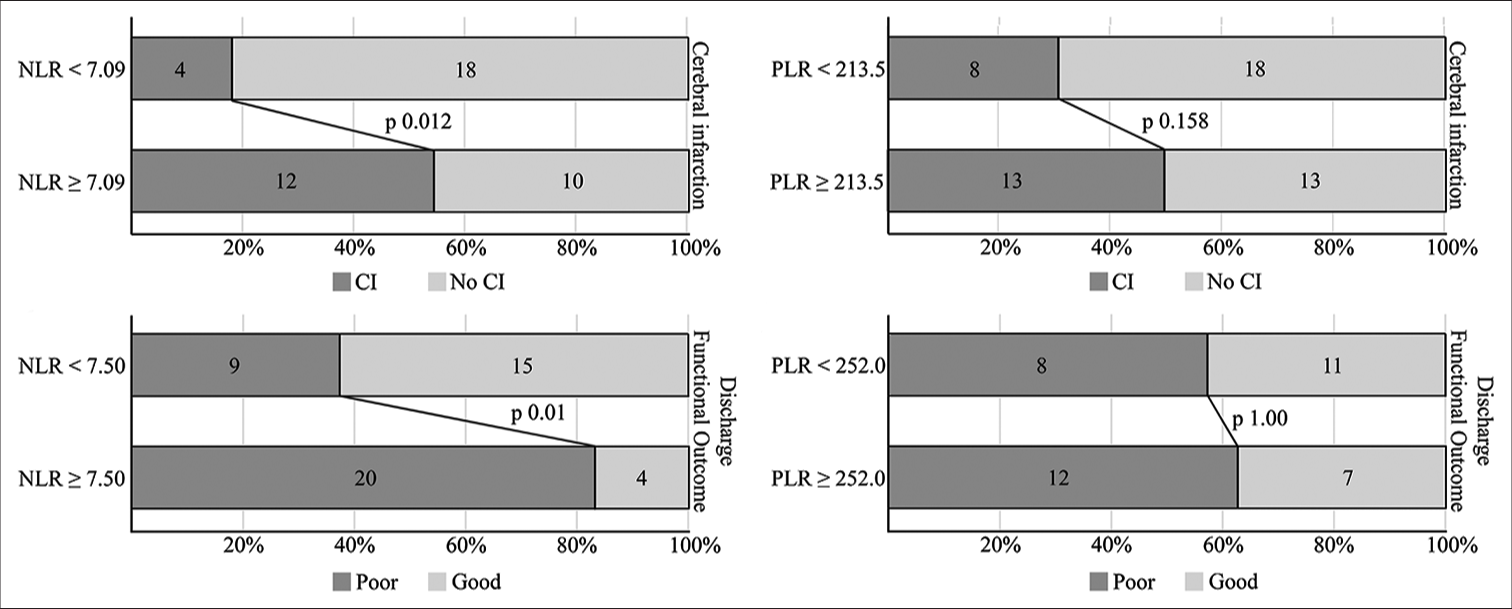
Results: Sixty-three patients were included in the study. NLR was independently associated with cerebral infarction (odds ratio, OR 1.197 [95% confidence interval, CI 1.027–1.395] per 1-point increment; P = 0.021) and poor discharge functional outcome (OR 1.175 [95% CI 1.036–1.334] per 1-point increment; P = 0.012). PLR did not significantly correlate with the outcomes. ROC analysis identified 7.09 as the cutoff for cerebral infarction and 7.50 for discharge functional outcome. Dichotomizing and performing PSM revealed that patients with NLR above the identified cutoff value significantly had more cerebral infarction and poor discharge functional outcome.
Conclusion: NLR demonstrated a good prognostic capability in Indonesian aSAH patients. More studies should be conducted to find the optimal cutoff value for each population.
Keywords: Biomarker, Cerebral infarction, Functional outcome, Neutrophil-lymphocyte-ratio, Subarachnoid hemorrhage
INTRODUCTION
Despite the significant advances in the management of aneurysmal subarachnoid hemorrhage (aSAH), its mortality remains as high as 20%.[
A good screening model is required to stratify those high-risk patients that would benefit from a preventive treatment while taking the risk of additional side effects. The widely used parameters, the Hunt and Hess scale, the World federation of neurosurgical societies (WFNS) scale, and the modified Fisher (mFisher) scale, only take into account the patient’s clinical and radiographical characteristics. With the elucidation of the complex biological process behind infarction,[
MATERIALS AND METHODS
Study population
We retrospectively collected the data for all aSAH patients admitted to our hospital between 2017 and 2021. Our hospital was one of the tertiary referral centers for neurosurgical patients in Indonesia. We excluded patients with non-aSAH, those aged <18 years old, those that had an infection on admission, and those that did not undergo either coiling/clipping procedure. Patients were diagnosed with a head computed tomography (CT) scan or magnetic resonance imaging (MRI) and supplemented by a CT angiographic study. This study was approved by the Local Ethics Committee at the Local Institutional Research Board. No informed consent was required due to the retrospective nature and exclusion of patients’ identifier.
Data collection
We collected patients’ admission baseline characteristics from the medical records. These included demographic data, emergency room findings (i.e., signs and symptoms, Glasgow coma scale [GCS], blood pressure, and WFNS scale), and laboratory parameters. The severity of aSAH was determined by the WFNS scale and was classified as severe (WFNS scale 3–5) and non-severe (WFNS scale 1–2). We only collected admission laboratory parameters. We used the full blood count differentials and platelet count to identify and calculate NLR and PLR. We also collected data on routine laboratory examinations such as hemoglobin, urea, and electrolytes. We did not plan to analyze other laboratory examinations such as random blood glucose, liver enzymes, lactate, or albumin because those examinations were not routinely performed in our center, so not all patients were examined for those parameters. We collected patients’ radiological images from our electronic database and used the mFisher scale to evaluate the bleeding extent. We collected patients’ discharge data from the medical records.
Imaging
The diagnosis of aSAH was established on admission using either a head CT scan or MRI. Non-aSAH was excluded using CT-angiography. We identified iatrogenic infarcts by radiologically examining patients 24–48 h after the procedure and differentiated them from infarcts that occurred afterward. During the patients’ stay, an additional radiographic evaluation could be ordered according to the judgment of the treating neurosurgeon. A final radiographical evaluation was done on all patients before their discharge.
During the data collection process, a radiologist blinded to the patient’s data performed another reading of the radiographic image to confirm the diagnosis of aSAH, grade them using the mFisher scale, and identify any intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH), or cerebral infarction. In our study, we followed the definition for cerebral infarction as proposed by Vergouwen et al.[
Clinical management
Patients diagnosed with aSAH underwent either a coiling or clipping procedure, according to the judgment of the treating neurosurgeon at the time. Standard supportive and symptomatic medical therapies were given to all patients along with prophylaxis nimodipine in the intensive care setting after the procedure. Patients were then transferred to the medical ward and discharged following stabilization and improvement of clinical condition.
Outcome evaluation
Cerebral infarction was the primary outcome of this study. In addition, we also evaluated patients’ discharge functional outcome. We used the modified Rankin scale (mRS), and we classified poor outcome at discharge as an mRS score of 3–6, representing functional dependence.
Statistical analysis
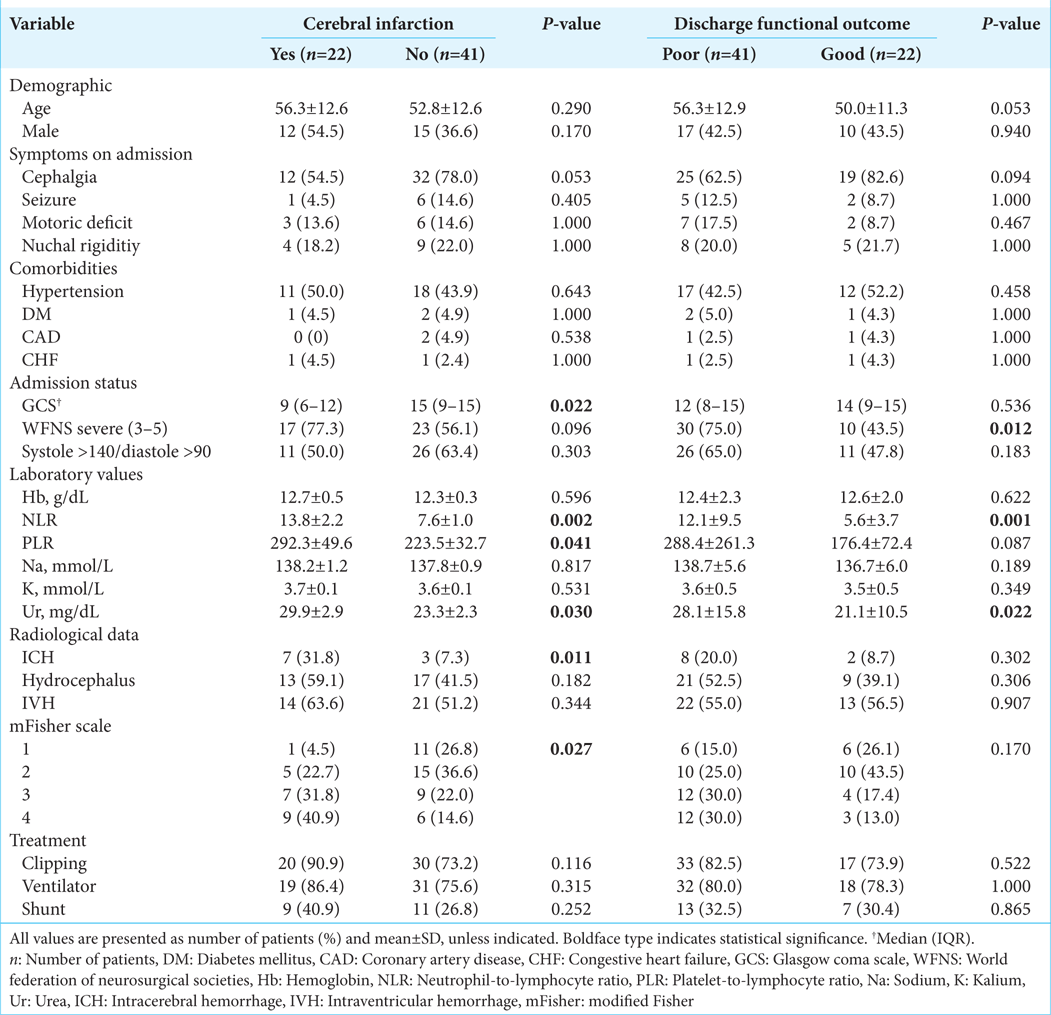
Patients’ baseline characteristics, clinical condition on admission, radiographic characteristics, and laboratory parameters were categorized according to the presence of cerebral infarction and discharge functional outcome. Categorical variables were compared using Chi-square or Fisher’s exact test. Continuous variables were analyzed for their distribution and were compared using Student’s t-test or Mann–Whitney U-test according to their distribution. Any variables showing statistical significance in the bivariate analysis were analyzed in a multivariate logistic regression model. We then performed a receiver operating characteristic (ROC) analysis to determine their respective cutoff value. All statistical significance was set at P < 0.05.
We then dichotomized the patient’s data according to the identified cutoff value. To reduce confounding bias, we performed a propensity score matching (PSM) with a 1:1 match ratio and caliper 0.15. We included demographic data such as age and sex, clinical parameters such as GCS, WFNS scale, blood pressure, ICH, IVH, mFisher scale, treatment (clipping or coiling), and those variables that were statistically significant in the initial multivariate analysis as covariates. Balance diagnostic was performed using p-value and standardized difference according to the formula outlined by Austin.[
RESULTS
Patient characteristics
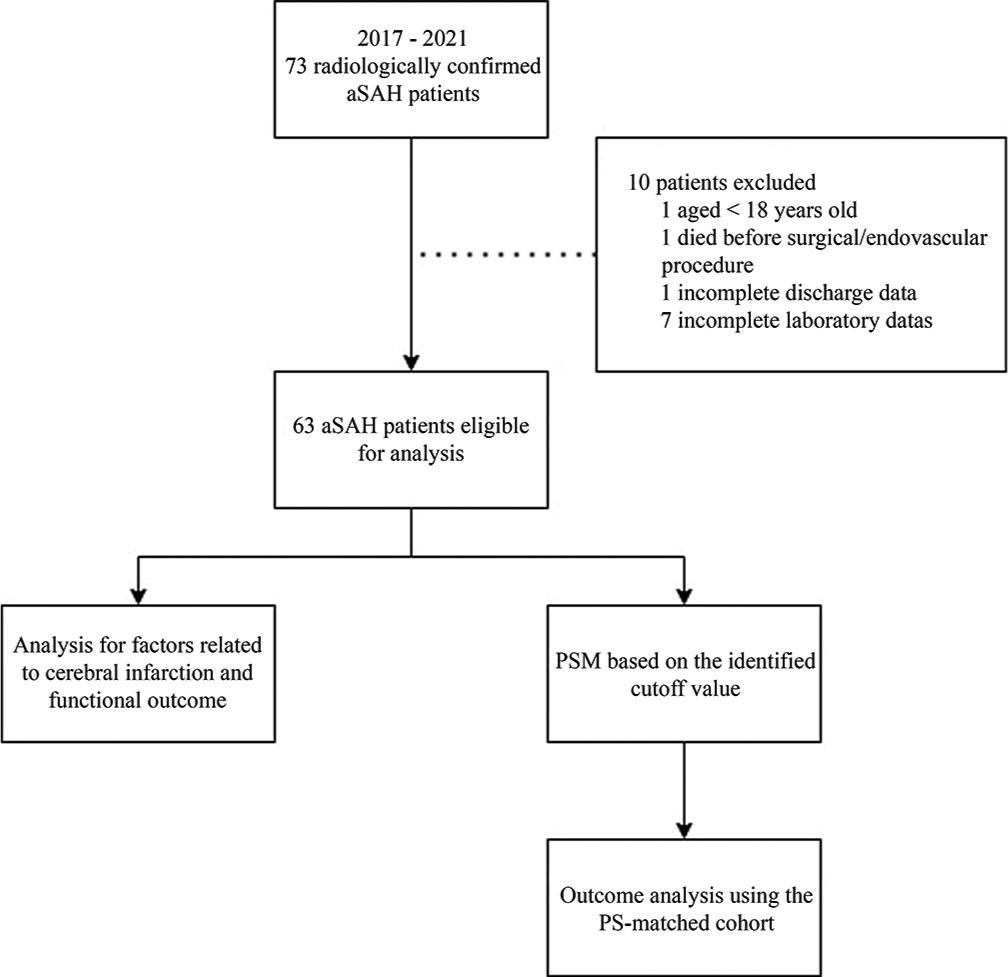
We identified 73 aSAH patients who underwent coiling or clipping procedures in our center between 2017 and 2021. We excluded seven patients due to incomplete laboratory data, one patient due to incomplete discharge data, one patient that died before treatment, and one adolescent patient [
Association of NLR and PLR with outcomes
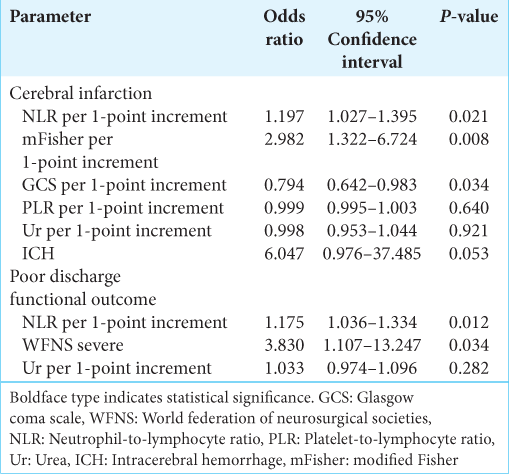
In the multivariate logistic regression [
We included NLR, severe WFNS score, and urea level in the model for poor discharge functional outcomes. We found that NLR was independently associated with discharge poor functional outcome (OR 1.175 [95% CI 1.036–1.334] per 1-point increment; P = 0.012). Severe WNFS scale was also independently associated with discharge poor functional outcome (OR 3.830 [95% CI 1.107–13.247]; P = 0.034).
ROC analysis
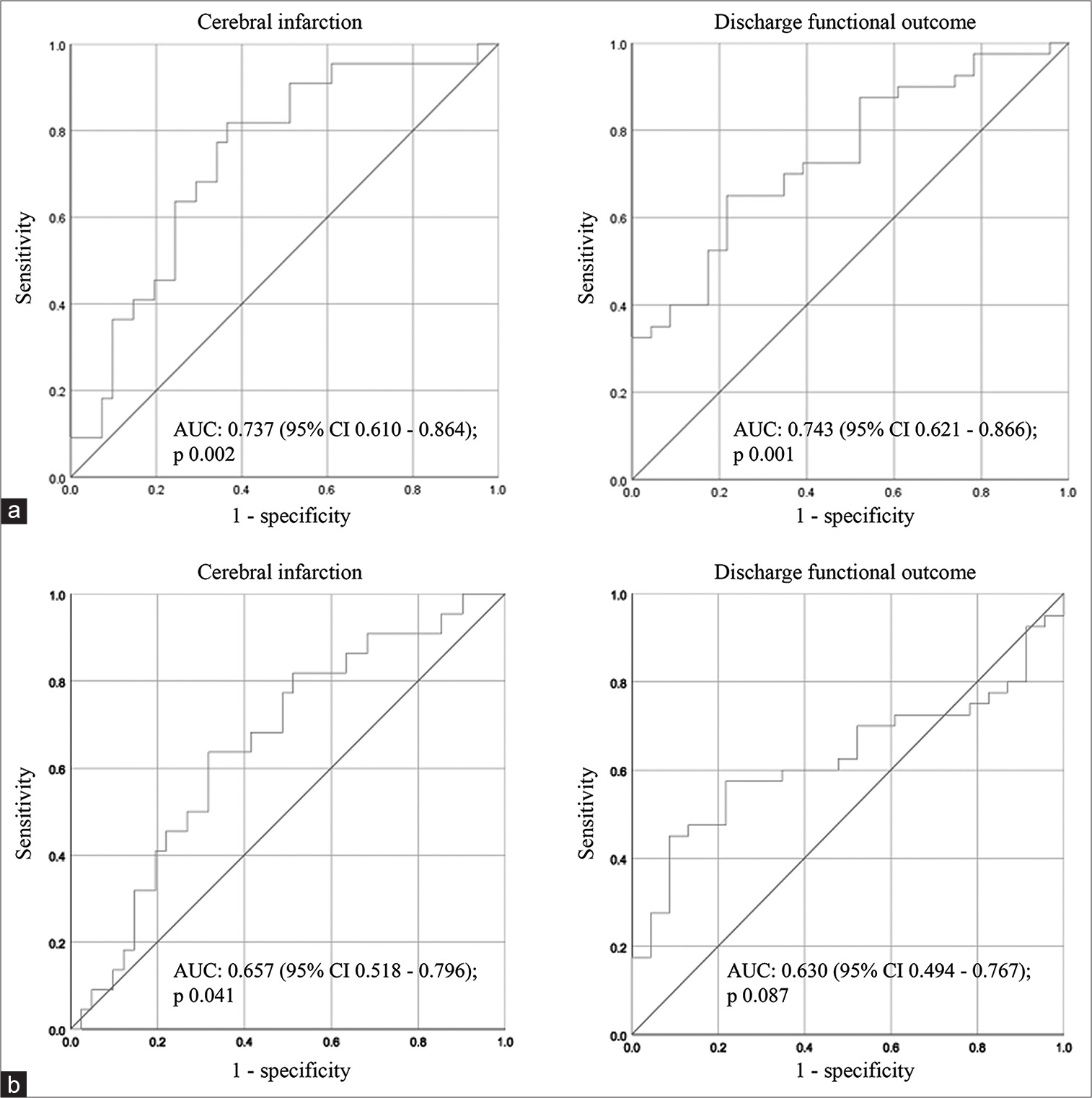
We performed an ROC analysis for NLR and PLR [
For PLR, the best cutoff value for predicting delayed cerebral ischemia (DCI) was 213.5 (Youden’s index 0.319; AUC 0.657 [95% CI 0.518–0.796]; P = 0.041). It had 63.6% sensitivity, 68.3% specificity, 51.9% PPV, and 77.8% NPV. For predicting poor discharge functional outcome, the best PLR cutoff value was 252.0 (Youden’s index 0.363; AUC 0.630 [95% CI 0.494–0.767]; P = 0.087). It had 45.0% sensitivity, 91.3% specificity, 90.1% PPV, and 47.1% NPV. However, the ROC analysis of PLR and poor discharge functional outcome did not reach statistical significance.
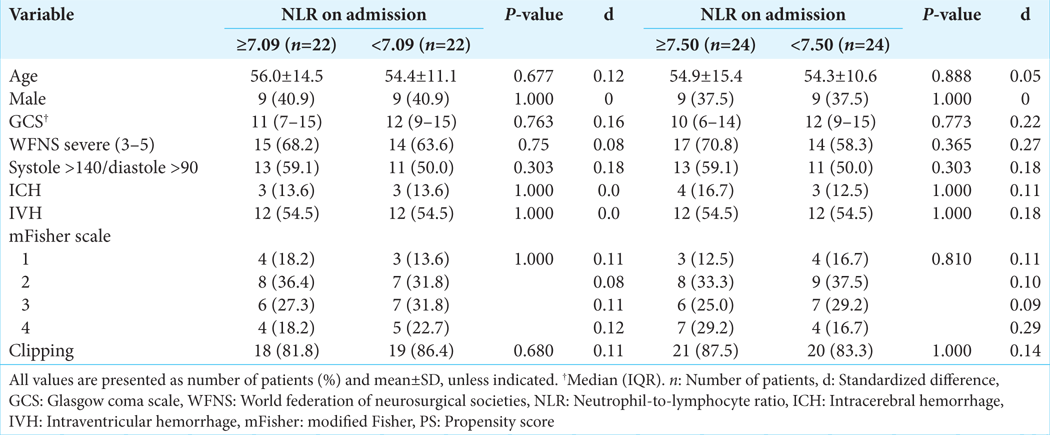
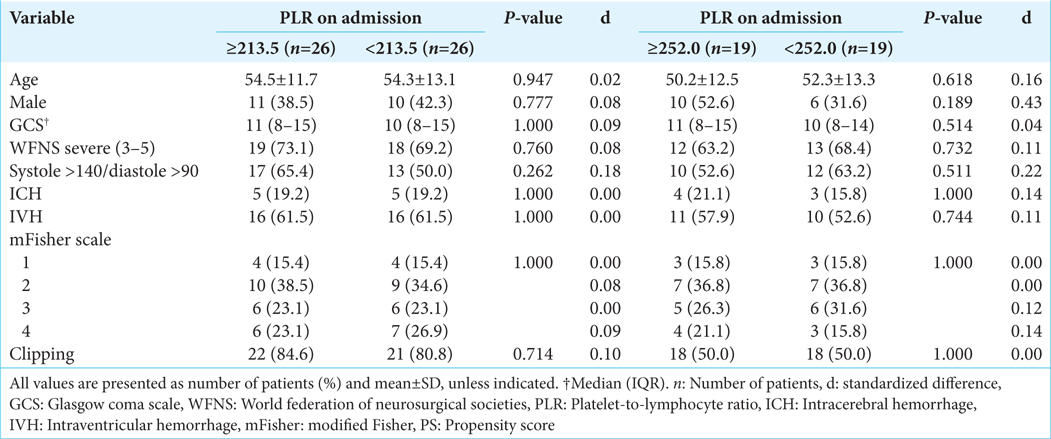
Outcomes in the PS-matched cohort
The balance diagnostic of the created PS-matched cohort are shown in
DISCUSSION
Based on the results of our literature search and several systematic reviews,[
Platelet has also been implicated in the development of cerebral infarction through the formation of microthrombi and increased inflammation level.[
We also found that a higher NLR value was independently associated with poor discharge functional outcome. Although we could only evaluate functional outcome on discharge, this result was in-line with other studies that evaluated outcome over a more extended period.[
We identified 7.09 and 7.50 as the optimal cutoff value for cerebral infarction and discharge functional outcome, respectively. Dichotomizing patients according to this cutoff value in the PS-matched cohort also showed a significantly higher proportion of patients in the NLR value group having the observed outcomes. The reported cutoff value from other studies ranged from 4.0 to 14.0, either for infarction or functional outcome.[
The difference between our cutoff value for cerebral infarction with those aforementioned studies could also be attributed to the difference in outcome criteria. Three studies used the term DCI, which focused more on focal neurological impairment and unexplainable decrease in consciousness,[
Our result supported the prognostic importance of NLR as a biomarker for cerebral infarction and poor functional outcomes. However, rather than replacing the widely used WFNS and mFisher scale, we suggested using NLR in combination with those two scales. As mentioned above, clazosentan and heparin have been studied for preventing cerebral vasospasm and infarction. Several clinical trials for clazosentan have produced conflicting results. The Clazosentan to Overcome Neurological Ischemia and Infarct Occurring After Subarachnoid Hemorrhage (CONSCIOUS)-3 trials demonstrated that clazosentan reduced vasospasm-related morbidity and all-cause mortality but did not affect the long-term functional outcome.[
Our study has several limitations. The retrospective and single-center nature of our study could have introduced confounding bias. We tried to minimize this by performing multivariate analysis and PSM and still found an independent association between NLR and cerebral infarction and functional outcome. However, unknown confounding bias could still have emerged and affected our findings. Another limitation is that we did not have our patients’ follow-up data after discharge, so we cannot analyze functional outcome over a longer period. Finally, since our hospital mainly served privately insured patients, this led to the small number of samples in our study, which could reduce the confidence in our result. Conducting a large-scale study in Indonesia is difficult as, currently, there is no national cohort on aSAH. However, building on our result, we hope that larger studies in Indonesia and other developing countries could be conducted in the future.
CONCLUSION
NLR is independently associated with cerebral infarction and poor discharge functional outcome. There is a significant disparity between studies regarding the reported cutoff value. As there is a possibility that NLR is affected by racial differences, we suggest that more research be conducted, especially in the developing countries. NLR’s low cost and ease would serve as an especially important prognostic marker in those countries. In addition, since prophylaxis treatment is possibly affected by patients’ severity, we suggest future research to incorporate NLR with WFNS and mFisher scale to classify aSAH patients’ severity better.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Khindi T, MacDonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010. 41: e519-36
2. Al-Mufti F, Amuluru K, Damodara N, Dodson V, Roh D, Agarwal S. Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurointerv Surg. 2019. 11: 1135-40
3. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011. 46: 399-424
4. Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014. 9: e112361
5. Bolton WS, Gharial PK, Akhunbay-Fudge C, Chumas P, Mathew RK, Anderson IA. Day 2 neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for prediction of delayed cerebral ischemia in subarachnoid hemorrhage. Neurosurg Focus. 2022. 52: E4
6. Cai L, Zeng H, Tan X, Wu X, Qian C, Chen G. The role of the blood neutrophil-to-lymphocyte ratio in aneurysmal subarachnoid hemorrhage. Front Neurol. 2021. 12: 671098
7. Chan V, Lindsay P, McQuiggan J, Zagorski B, Hill MD, O’Kelly C. Declining admission and mortality rates for subarachnoid hemorrhage in Canada between 2004 and 2015. Stroke. 2018. 50: 181-4
8. Dodd WS, Laurent D, Dumont AS, Hasan DM, Jabbour PM, Starke RM. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: A review. J Am Heart Assoc. 2021. 10: e021845
9. Endo H, Hagihara Y, Kimura N, Takizawa K, Niizuma K, Togo O. Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: Two randomized phase 3 trials in Japanese patients. J Neurosurg. 2022. 137: 1707-17
10. Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition?. Stroke. 2009. 40: 1963-8
11. Giede-Jeppe A, Reichl J, Sprügel MI, Lücking H, Hoelter P, Eyüpoglu IY. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019. 132: 400-7
12. Guo Y, Liu J, Zeng H, Cai L, Wang T, Wu X. Neutrophil to lymphocyte ratio predicting poor outcome after aneurysmal subarachnoid hemorrhage: A retrospective study and updated meta-analysis. Front Immunol. 2022. 13: 962760
13. Gusdon AM, Thompson CB, Quirk K, Mayasi YM, Avadhani R, Awad IA. CSF and serum inflammatory response and association with outcomes in spontaneous intracerebral hemorrhage with intraventricular extension: An analysis of the clear-III Trial. J Neuroinflammation. 2021. 18: 179
14. Hanhai Z, Bin Q, Shengjun Z, Jingbo L, Yinghan G, Lingxin C. Neutrophil extracellular traps, released from neutrophil, promote microglia inflammation and contribute to poor outcome in subarachnoid hemorrhage. Aging (Albany NY). 2021. 13: 13108-23
15. Höllig A, Stoffel-Wagner B, Clusmann H, Veldeman M, Schubert GA, Coburn M. Time courses of inflammatory markers after aneurysmal subarachnoid hemorrhage and their possible relevance for future studies. Front Neurol. 2017. 8: 694
16. Klein RS, Hunter CA. Protective and pathological immunity during central nervous system infections. Immunity. 2017. 46: 891-909
17. Li W, Hou M, Ding Z, Liu X, Shao Y, Li X. Prognostic value of neutrophil-to-lymphocyte ratio in stroke: A systematic review and meta-analysis. Front Neurol. 2021. 12: 686983
18. Lukito PP, Lie H, Helsa K, July J. Heparin in the treatment of aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurosurg Focus. 2022. 52: E9
19. MacDonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012. 43: 1463-9
20. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017. 389: 655-66
21. Mayer SA, Aldrich EF, Bruder N, Hmissi A, Macdonald RL, Viarasilpa T. Thick and diffuse subarachnoid blood as a treatment effect modifier of clazosentan after subarachnoid hemorrhage. Stroke. 2019. 50: 2738-44
22. McMahon CJ, Hopkins S, Vail A, King AT, Smith D, Illingworth KJ. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg. 2013. 5: 512-7
23. Raatikainen E, Kiiski H, Kuitunen A, Junttila E, Huhtala H, Ronkainen A. Platelet count is not associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as defined by the 2010 consensus definition. J Neurol Sci. 2022. 436: 120227
24. Shi M, Yang C, Tang QW, Xiao LF, Chen ZH, Zhao WY. The prognostic value of neutrophil-to-lymphocyte ratio in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis of observational studies. Front Neurol. 2021. 12: 745560
25. Tao C, Wang J, Hu X, Ma J, Li H, You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017. 26: 393-401
26. Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J Neurochem. 2016. 139: 91-114
27. Vandooren J, Van Damme J, Opdenakker G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog Brain Res. 2014. 214: 193-206
28. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010. 41: 2391-5
29. Wang JY, Zhang XT, Wang JQ, Wang CY, Zheng WL, Pan ZM. Admission neutrophil-lymphocyte ratio predicts rebleeding following aneurismal subarachnoid hemorrhage. World Neurosurg. 2020. 138: e317-22
30. Wu Y, He Q, Wei Y, Zhu J, He Z, Zhang X. The association of neutrophil-to-lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: Possible involvement of cerebral blood perfusion. Neuropsychiatr Dis Treat. 2019. 15: 1001-7
31. Yi HJ, Lee DH, Sung JH. Inflammation-based Scores are associated with the prognosis of patients with aneurysmal subarachnoid hemorrhage after neuro-intervention. Curr Neurovasc Res. 2020. 17: 676-85
32. Yun S, Yi HJ, Lee DH, Sung JH. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2021. 30: 105861
33. Zhang B, Lin L, Yuan F, Song G, Chang Q, Wu Z. Clinical application values of neutrophil-to-lymphocyte ratio in intracranial aneurysms. Aging (Albany NY). 2021. 13: 5250-62
34. Zhang P, Li Y, Zhang H, Wang X, Dong L, Yan Z. Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg. 2020. p. 1-7