- Department of Neurosurgery, Tsukuba Memorial Hospital, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Neurology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_974_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michihide Kajita1, Kiyoyuki Yanaka1, Ken Akimoto1,2, Hitoshi Aiyama1, Kazuhiro Ishii3, Eiichi Ishikawa2. Nonaneurysmal subarachnoid hemorrhage associated with COVID-19 infection: A case report. 11-Nov-2022;13:524
How to cite this URL: Michihide Kajita1, Kiyoyuki Yanaka1, Ken Akimoto1,2, Hitoshi Aiyama1, Kazuhiro Ishii3, Eiichi Ishikawa2. Nonaneurysmal subarachnoid hemorrhage associated with COVID-19 infection: A case report. 11-Nov-2022;13:524. Available from: https://surgicalneurologyint.com/surgicalint-articles/11992/
Abstract
Background: Most coronavirus disease 2019 (COVID-19)-related cerebrovascular disorders are ischemic while hemorrhagic disorders are rarely reported. Among these, subarachnoid hemorrhage (SAH) is very rarely reported and nonaneurysmal SAH has been reported in only about a dozen cases. Here, we report a case of nonaneurysmal SAH as the only clinical manifestation of COVID-19 infection. In addition, we reviewed and analyzed the literature data on cases of nonaneurysmal SAH caused by COVID-19 infection.
Case Description: A 50-year-old woman presented to an emergency department with a sudden headache, right hemiparesis, and consciousness disturbance. At that time, no fever or respiratory failure was observed. Laboratory data were within normal values but the rapid antigen test for COVID-19 on admission was positive, resulting in a diagnosis of COVID-19 infection. Computed tomograms (CTs) showed bilateral convexal SAH with a hematoma but three-dimensional CT angiograms showed no obvious sources, such as a cerebral aneurysm. Therefore, the patient was diagnosed with nonaneurysmal SAH associated with COVID-19 infection. With conservative treatment, consciousness level and hemiparesis both improved gradually until transfer for continued rehabilitation. Approximately 12 weeks after onset, the patient was discharged with only mild cognitive impairment. During the entire course of the disease, the headache, hemiparesis, and mild cognitive impairment due to nonaneurysmal SAH with small hematoma were the only abnormalities experienced.
Conclusion: Since COVID-19 infection can cause nonaneurysmal hemorrhaging, it should be considered (even in the absence of characteristic infectious or respiratory symptoms of COVID-19) when atypical hemorrhage distribution is seen as in our case.
Keywords: Coronavirus disease 2019, Nonaneurysmal subarachnoid hemorrhage, Stroke
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 infection can affect the respiratory tract and other organs, including the brain, and is considered a risk factor for multiorgan ischemic and hemorrhagic diseases.[
A higher incidence of aneurysmal subarachnoid hemorrhage (SAH) has been reported in COVID-19 patients, but whether this increase is incidental or related is controversial.[
Here, we describe a case of nonaneurysmal SAH with small hematoma, resulting in headache, hemiparesis, and mild cognitive impairment as the only abnormalities related to COVID-19 infection. We also review previously reported cases of nonaneurysmal SAH associated with COVID-19 infection and discuss their characteristics.
CASE DESCRIPTION
A 50-year-old woman came to our emergency department complaining of a sudden headache, right hemiparesis, and loss of consciousness. She had no significant or relevant medical history other than a previous, small, and unruptured cerebral aneurysm for which she had undergone regular imaging.
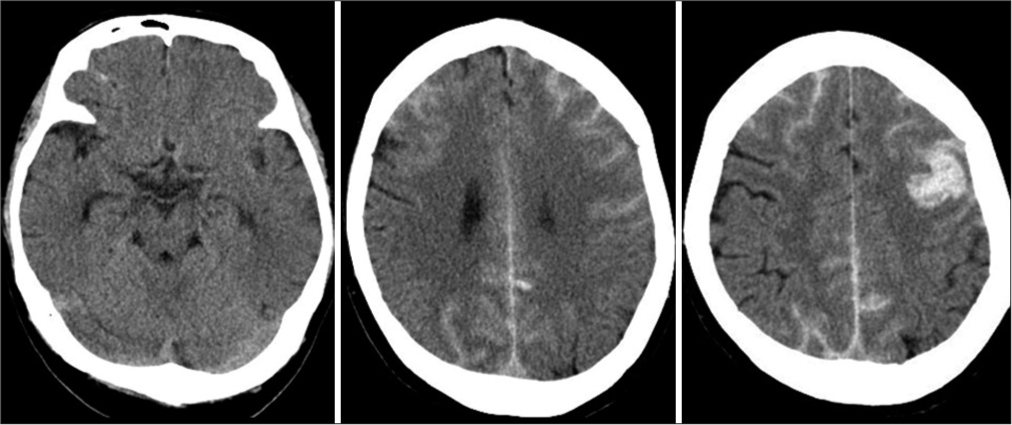
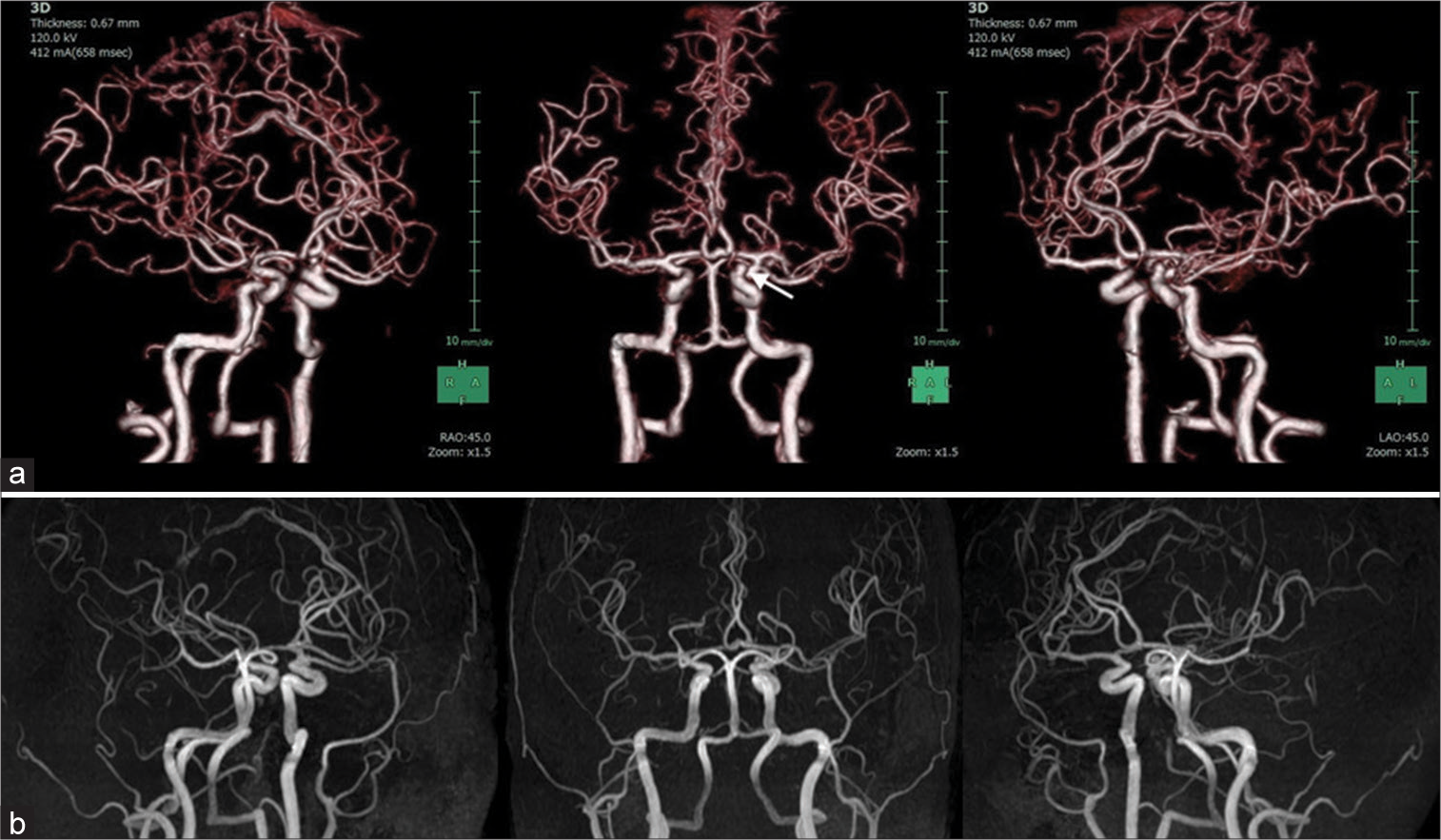
On admission, physical examination revealed a body temperature of 98.2°F, respiratory rate of 20 breaths/min, peripheral oxygen saturation of 98% on room air, and blood pressure of 147/86 mmHg. The right hemiparesis with the upper extremity predominance and mild consciousness disturbance was observed. General laboratory findings, including thyroid function and factors associated with collagen diseases, were within normal ranges, and there were no inflammatory findings or coagulation abnormalities (PT: 11.1 s, APTT: 28.7 s, and D-dimer: 0.7 μg/dl). Our institution performs antigen testing in all patients who require hospitalization to prevent nosocomial infection; this patient tested positive by the rapid antigen test and was diagnosed with COVID-19. The chest X-ray was normal, and the chest computed tomogram (CT) showed no pneumonia. CT of the brain showed SAH over a wide area of the bilateral convexal regions and a hematoma in the left frontal sulci [
Although no respiratory symptoms were present, remdesivir was administered for the viral infection, as SAH is a likely indicator of severe COVID-19. In addition, conservative treatment, including hemostatic agent administration, osmotic therapy, blood pressure control, and continuous rehabilitation, gradually improved neurological deficits such as consciousness disturbance and hemiparesis. Magnetic resonance (MR) angiograms and contrast-enhanced MR images 14 days after admission showed no vascular or neoplastic lesions suggestive of a hemorrhage source. Approximately 12 weeks after onset, the patient was discharged with only mild cognitive impairment. During hospitalization, the patient had no respiratory symptoms or recurrent hemorrhagic/ ischemic strokes. No hemorrhage sources were seen on MR images at the time of discharge [
DISCUSSION
SAH is primarily caused by trauma or ruptured cerebral aneurysms but other causes include rupture of abnormal vessels (such as arteriovenous malformations and arteriovenous fistulas), vasculitis, tumors, arterial dissection, moyamoya disease, coagulation abnormalities, cocaine abuse, sickle cell disease, infection, perimesencephalic nonaneurysmal SAH, cerebral venous thrombosis, reversible cerebral vasoconstriction syndrome, posterior reversible encephalopathy syndrome, cerebral amyloid angiopathy, and others.[
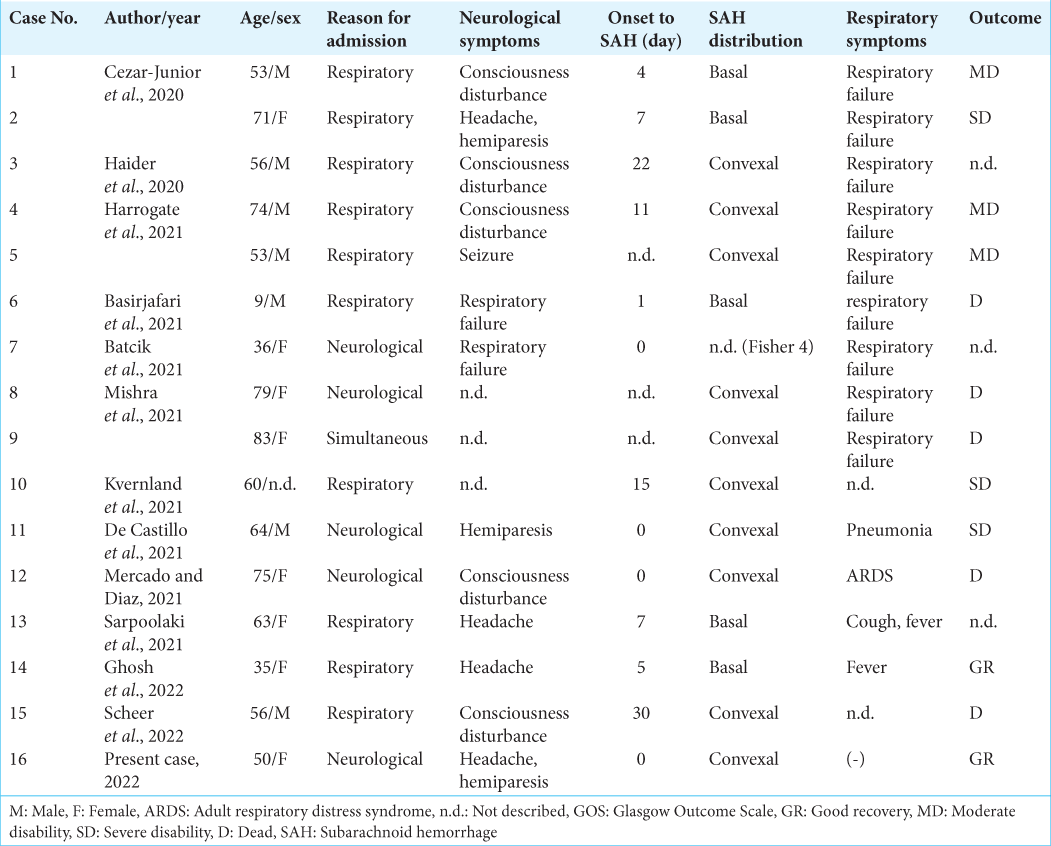
A literature review revealed 16 cases of nonaneurysmal SAH associated with COVID-19 infection, including ours [
Among these cases, preceding neurological symptoms were extant in 5 cases (31.3%) and respiratory symptoms were seen in 11 cases (68.8%), indicating the challenge of precise COVID-19 infection diagnosis based on chief complaints in about 1/3 of the cases. Of the total cases, eight exhibited severe respiratory failure requiring ventilation but, even with preceding neurological symptoms, severe respiratory failure was later observed in 3 out of 5 patients (60.0%). Therefore, COVID-19 infection respiratory status requires continuous monitoring even if no initial abnormalities are observed.
Some cases presented stroke-specific symptoms, such as consciousness disturbance and hemiplegia, while others had only nonspecific symptoms such as headache. Headache is a common symptom of infectious diseases but the possibility of nonaneurysmal SAH should always be considered. As reported intervals from infection to nonaneurysmal SAH onset ranged from 0 to 30 days (average of 7.8 ± 9.4 days), COVID-19 patients could develop SAH at approximately 2 weeks after infection.
In addition, SAH distribution patterns in COVID-19 patients often differed from those caused by ruptured cerebral aneurysms. Ruptured cerebral aneurysm patterns for SAH vary depending on location but they generally spread in the basal cistern. In contrast, COVID-19 patients have a more peripheral hemorrhage distribution in the cortical areas. Several hemorrhagic mechanisms focused on vascular endothelial damage have been postulated for the etiology of SAH in COVID-19, including vascular barrier damage associated with hypercytokinemia, virus-induced endothelial damage, coagulopathy, and immune thrombocytopenia.[
Some COVID-19 patients also had multiple cerebral infarctions.[
Of the 13 patients with known prognoses, 5 (38.5%) had a favorable outcome, while 3 (23.1%) were severe disability and 5 (38.5%) died. The high mortality rate compared to typical COVID-19 infection (8.3~10%)[
Numerous reports of viral infections and nonaneurysmal SAH have been associated with COVID-19 caused by SARS-CoV-2,[
CONCLUSION
In this report, we described a case of COVID-19 infection where nonaneurysmal SAH was an isolated symptom. There are two important lessons to be learned from this case. First, COVID-19 infection can cause nonaneurysmal SAH, which often has atypical hematoma distribution. Second, COVID-19 infection should be suspected in patients with atypical SAH distribution, even without typical infectious signs (such as fever or respiratory symptoms).
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Basirjafari S, Rafiee M, Shahhosseini B, Mohammadi M, Neshin SA, Zarei M. Association of pediatric COVID-19 and subarachnoid hemorrhage. J Med Virol. 2021. 93: 658-60
2. Batcik OE, Kanat A, Cankay TU, Ozturk G, Kazancıoglu L, Kazdal H. COVID-19 infection produces subarachnoid hemorrhage; Acting now to understand its cause: A short communication. Clin Neurol Neurosurg. 2021. 202: 106495
3. Cezar-Junior AB, Faquini IV, Silva JL, de Carvalho Junior EV, Lemos LE, Filho JB. Subarachnoid hemorrhage and COVID-19: Association or coincidence?. Medicine (Baltimore). 2020. 99: e23862
4. de Castillo LL, Diestro JD, Ignacio KH, Separa KJ, Pasco PM, Franks MC. Concurrent acute ischemic stroke and non-aneurysmal subarachnoid hemorrhage in COVID-19. Can J Neurol Sci. 2021. 48: 587-8
5. Ghosh R, Roy D, Ray A, Mandal A, Das S, Pal SK. Nonaneurysmal subarachnoid hemorrhage in COVID-19: A case report and review of literature. Med Res Arch. 2022. 10: 2673
6. Haider A, Schmitt C, Greim CA. Multiorgan point-of-care ultrasound in a patient with coronavirus disease 2019 pneumonia complicated by subarachnoid hemorrhage and pulmonary embolism. A A Pract 2020. ;. 14: e01357
7. Harrogate S, Mortimer A, Burrows L, Fiddes B, Thomas I, Rice CM. Non-aneurysmal subarachnoid haemorrhage in COVID-19. Neuroradiology. 2021. 63: 149-52
8. Iba T, Levi M, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Semin Thromb Hemost. 2020. 46: 89-95
9. Ildan F, Tuna M, Erman T, Göçer AI, Cetinalp E. Prognosis and prognostic factors in nonaneurysmal perimesencephalic hemorrhage: A follow-up study in 29 patients. Surg Neurol. 2002. 57: 160-5 discussion 165-6
10. Khan F, Sharma N, Ud Din M, Shirke S, Abbas S. Convexal subarachnoid hemorrhage caused by infective endocarditis in a patient with advanced Human Immunodeficiency Virus (HIV): The culprits and bystanders. Am J Case Rep. 2021. 22: e931376
11. Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Güresir E. Non-aneurysmal non-traumatic subarachnoid hemorrhage: Patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol. 2014. 14: 140
12. Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A. Anticoagulation use and hemorrhagic stroke in SARSCoV-2 patients treated at a New York healthcare system. Neurocrit Care. 2021. 34: 748-59
13. Marushima A, Yanaka K, Matsuki T, Kojima H, Nose T. Subarachnoid hemorrhage not due to ruptured aneurysm in moyamoya disease. J Clin Neurosci. 2006. 13: 146-9
14. Matsumaru Y, Yanaka K, Muroi A, Sato H, Kamezaki T, Nose T. Significance of a small bulge on the basilar artery in patients with perimesencephalic nonaneurysmal subarachnoid hemorrhage. Report of two cases. J Neurosurg. 2003. 98: 426-9
15. Matsumoto S, Matsumoto E. A 20-month-old girl with fever, seizures, hemiparesis, and brain lesions requiring a diagnostic brain biopsy. Semin Pediatr Neurol. 2018. 26: 80-2
16. Mercado ES, Diaz JB. Spontaneous non-aneurysmal subarachnoid hemorrhage as initial presentation of COVID-19: A case report. Med Rep Case Stud. 2021. 6: 224
17. Mishra S, Choueka M, Wang Q, Hu C, Visone S, Silver M. Intracranial hemorrhage in COVID-19 patients. J Stroke Cerebrovasc Dis. 2021. 30: 105603
18. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021. 16: 137-49
19. Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: The global COVID-19 stroke registry. Stroke. 2020. 51: e254-8
20. Renou P, Tourdias T, Fleury O, Debruxelles S, Rouanet F, Sibon I. Atraumatic nonaneurysmal sulcal subarachnoid hemorrhages: A diagnostic workup based on a case series. Cerebrovasc Dis. 2012. 34: 147-52
21. Rios J, Félix C, Proença P, Malaia L, Nzwalo H. Ischemic stroke and subarachnoid hemorrhage following epstein-barr virus infection. J Neurosci Rural Pract. 2020. 11: 680-1
22. Rinkel GJ, Wijdicks EF, Hasan D, Kienstra GE, Franke CL, Hageman LM. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet. 1991. 338: 964-8
23. Sarpoolaki MK, Iranmehr A, Bitaraf MA, Naderi S, Namvar M. Non-aneurysmal perimesencephalic subarachnoid hemorrhage in a COVID-19 patient: Case report and review on subarachnoid hemorrhage patterns in COVID-19. Iran J Neurosurg. 2021. 7: 213-8
24. Scheer M, Harder A, Wagner S, Ibe R, Prell J, Scheller C. Case report of a fulminant non-aneurysmal convexity subarachnoid hemorrhage after COVID-19. Interdiscip Neurosurg. 2022. 27: 101437
25. Silvestrini M, Floris R, Tagliati M, Stanzione P, Sancesario G. Spontaneous subarachnoid hemorrhage in an HIV patient. Ital J Neurol Sci. 1990. 11: 493-5
26. Tonomura Y, Kataoka H, Yata N, Kawahara M, Okuchi K, Ueno S. A successfully treated case of herpes simplex encephalitis complicated by subarachnoid bleeding: A case report. J Med Case Rep. 2010. 4: 310
27. VanderVeen N, Nguyen N, Hoang K, Parviz J, Khan T, Zhen A. Encephalitis with coinfection by Jamestown canyon virus (JCV) and varicella zoster virus (VZV). IDCases. 2020. 22: e00966
28. Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020. 382: e43