- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan,
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka, Japan,
- Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan,
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan.
Correspondence Address:
Nobutaka Mukae
Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan,
DOI:10.25259/SNI_709_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keisuke Abe1, Nobutaka Mukae1, Takato Morioka2, Yuhei Sangatsuda1, Ayumi Sakata3, Satoshi O. Suzuki4, Masahiro Mizoguchi1. Nonconvulsive status epilepticus associated with Alzheimer’s disease mimicking symptomatic focal epilepsy following the resection of a frontal parasagittal meningioma. 29-Dec-2020;11:469
How to cite this URL: Keisuke Abe1, Nobutaka Mukae1, Takato Morioka2, Yuhei Sangatsuda1, Ayumi Sakata3, Satoshi O. Suzuki4, Masahiro Mizoguchi1. Nonconvulsive status epilepticus associated with Alzheimer’s disease mimicking symptomatic focal epilepsy following the resection of a frontal parasagittal meningioma. 29-Dec-2020;11:469. Available from: https://surgicalneurologyint.com/surgicalint-articles/10499/
Abstract
Background: Epilepsies are frequent in patients with Alzheimer’s disease (AD); however, epilepsies in AD can easily go unrecognized because they usually present as focal impaired awareness seizures or nonconvulsive status epilepticus (NCSE) and can overlap with other symptoms of AD.
Case Description: We performed an epilepsy surgery in a 69-year-old woman with progressive cognitive impairment and consciousness disorder, who was diagnosed with focal NCSE related to the resected meningioma in the right frontal parasagittal region. Intraoperative electrocorticography revealed localized periodic paroxysmal discharges with beta and gamma activities in the neighboring cortex where the meningioma existed. The histopathological diagnosis of AD was first made from the resected epileptogenic cortex.
Conclusion: Even when there is a suspected epileptogenic lesion that could cause focal NCSE, AD should be ruled out in elderly patients with progressive cognitive decline.
Keywords: Alzheimer’s disease, Electrocorticography, Hydrocephalus, Meningioma, Nonconvulsive status epilepticus
INTRODUCTION
It is well known that epilepsies are more frequent in patients with Alzheimer’s disease (AD) than in those with non-AD dementias. An estimated 10–20% of patients with AD have clinically obvious epilepsies; however, in clinical practice, epilepsies in patients with AD can easily go unrecognized because they can present as focal impaired awareness seizures or nonconvulsive status epilepticus (NCSE) and can overlap with other symptoms of AD.[
On the other hand, we often encounter epileptic seizure after brain tumor surgery, which can also present as NCSE. When the patient after brain tumor surgery has developed NCSE and cognitive decline, the AD can be overlooked while the patient had AD as underlying disease. We should be aware of the possibility of underlying AD.
We performed an epilepsy surgery in elderly patient with progressive cognitive impairment and consciousness disorder, who was initially diagnosed with focal NCSE related to the resected meningioma in the right frontal parasagittal region. The histopathological diagnosis of AD was first made from the resected epileptogenic cortex, confirmed by intraoperative electrocorticography (ECoG). We report the detailed clinical course of this patient.
CASE REPORT
Sixty-nine-year-old women had been experiencing an 8-month slowly progressive decline in executive functions and language domains, which lead to difficulty in writing, calculating, and activities in daily living. On the Wechsler Adult Intelligence Scale-III, her intelligence quotient (IQ) levels were mildly impaired: verbal IQ = 82, performance IQ = 52, and full scale IQ = 64. Her Hasegawa Dementia Scale-Revised (HDS-R)[
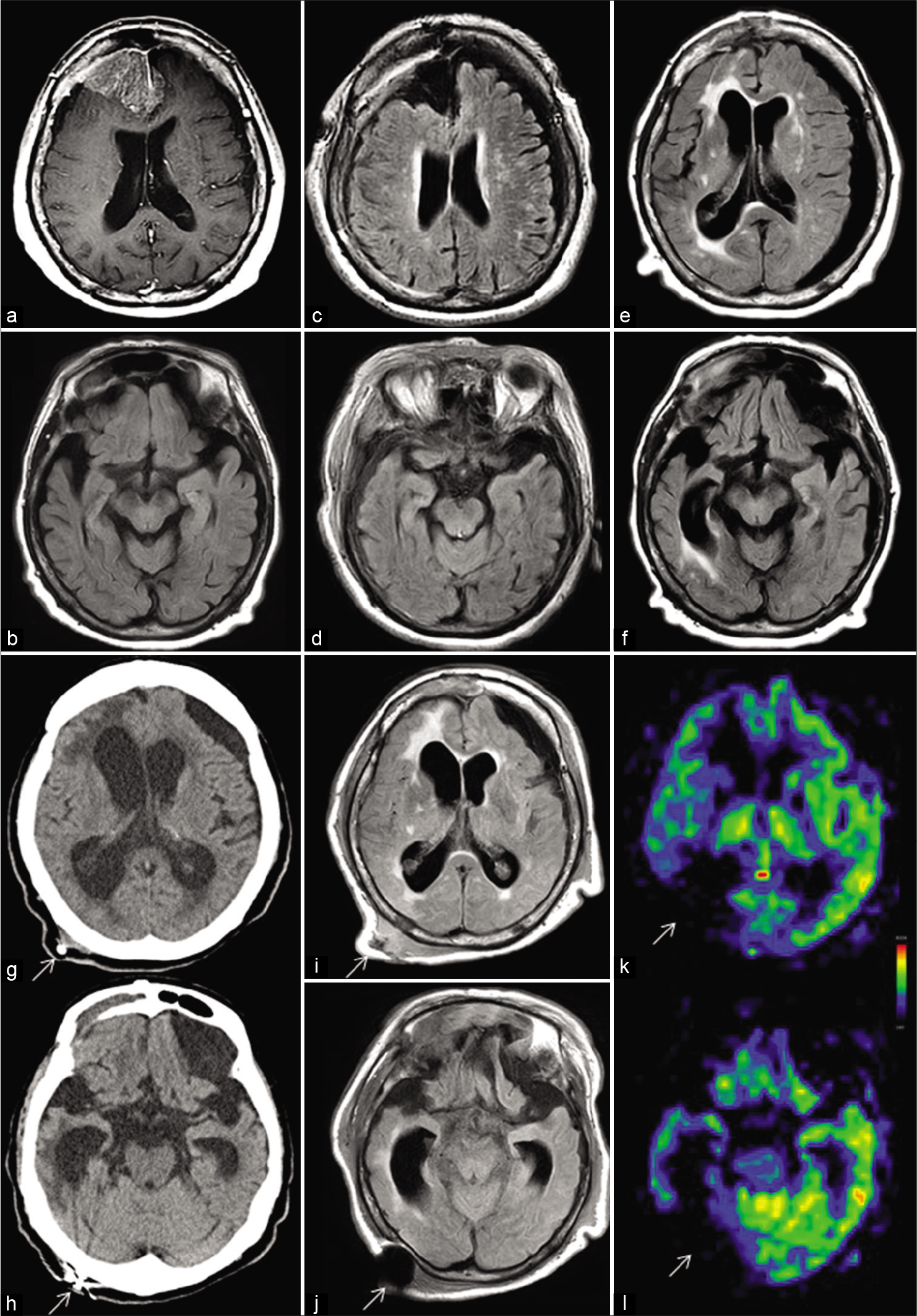
Figure 1:
(a and b) Preoperative magnetic resonance (MR) images. (a) T1-weighted MR image with gadolinium-DTPA enhancement demonstrates a large parasagittal meningioma in the right frontal region extending to the left side. (b) On MR image with fluid-attenuated inversion recovery sequence (FLAIR), mild atrophy of the cerebrum including the hippocampus is noted. (c and d) FLAIR images immediately after the surgery confirm the total removal of the meningioma through a right frontal craniotomy, without causing damage to the underlying cortex. (e and f) FLAIR images 3 months postoperatively reveal a subdural hematoma on the left side and marked ventricular enlargement. Bilateral hippocampal atrophy is more evident than that before tumor surgery. In particular, the right hippocampus is markedly atrophied, with marked enlargement of the right inferior horn. Periventricular hyperintensity, especially on the right side, is also observed. (g and h) Computed tomography image 6 months postoperatively shows progressive ventriculomegaly, while the left subdural hematoma is decreased in size. White arrows indicate the ventriculoperitoneal (VP) shunt. (i and j) FLAIR images, after changing the setting pressure of the VP shunt system, show that the ventricular size is well controlled. Atrophy of the cerebrum, including the hippocampus, and periventricular hyperintensity, predominantly on the right side, are apparent. White arrows indicate artifacts caused by the VP shunt system. (k and l) Perfusion MR with arterial spin labeling demonstrates a markedly decreased signal in the right cerebral cortex. White arrows indicate artifacts caused by the VP shunt system.
However, postoperatively, her cognitive dysfunction slowly deteriorated rather than improved. Three months postoperatively, she developed dysarthria, gait disturbance, and urinary incontinence and was readmitted. Her HDS-R score dropped to 15 of 30 points. MRI revealed ventriculomegaly and a subdural hematoma on the left side [
Six months after the first craniotomy, she developed a drowsy state. Computed tomography showed progressive ventricular enlargement [
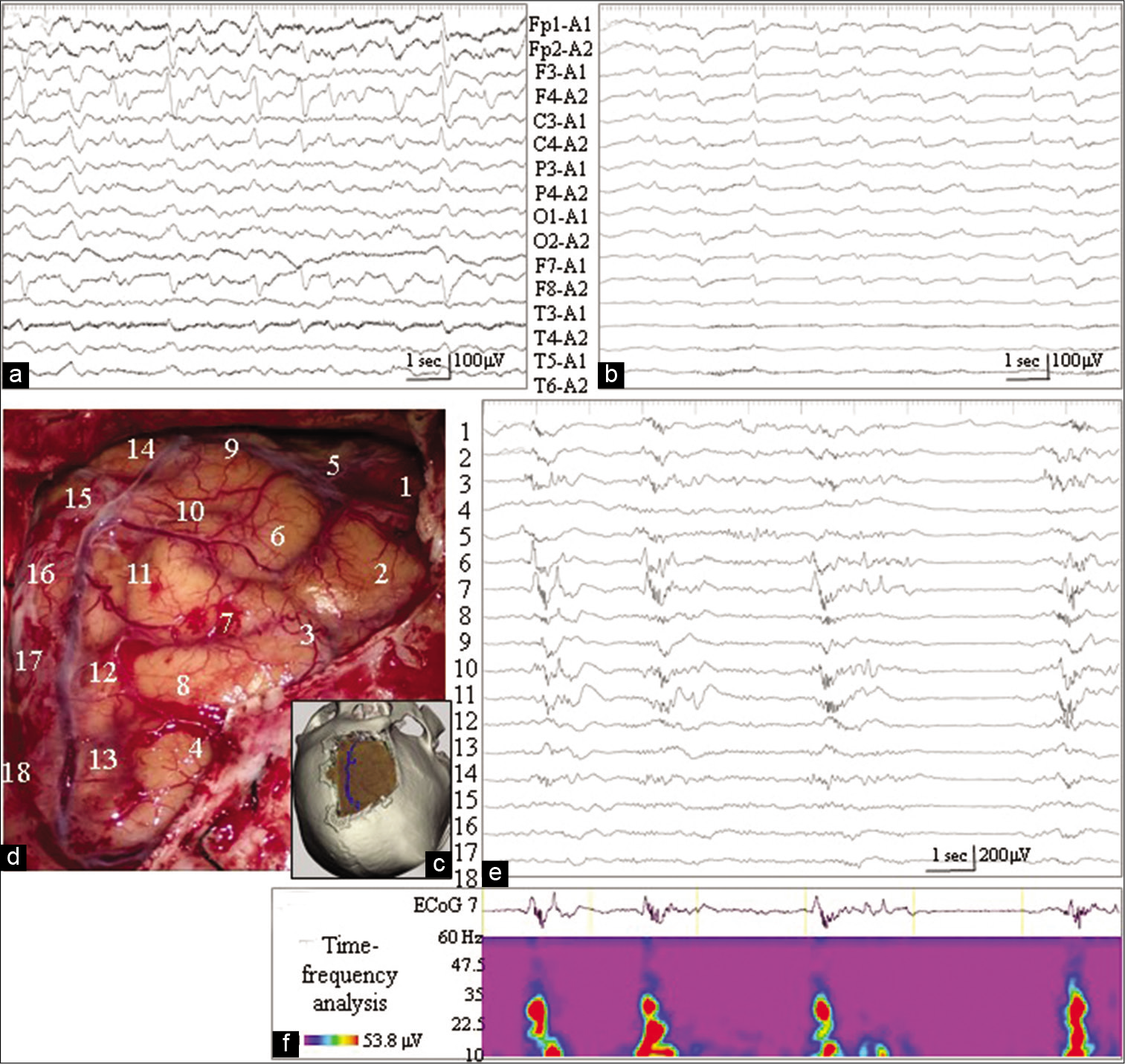
Figure 2:
(a) Electroencephalography (EEG) 3 months postoperatively reveals repetitive paroxysmal discharges in the right frontal region (F4 of the International EEG 10–20 System). (b) Continuous EEG monitoring during barbiturate coma therapy, 6 months after tumor removal, demonstrates periodic paroxysmal discharges, especially in the right frontal region. (c-f) Intraoperative findings during epilepsy surgery. (c) Three-dimensional reconstruction of the computed tomography scan demonstrates the extent of the craniotomy. (d) The position and number of the subdural electrodes are placed on the intraoperative photographs, shown in an orientation matched with (c). (e) Intraoperative electrocorticography (ECoG) reveals periodic paroxysmal discharges with the maximal amplitude of electrode No. 7. Fast-wave activities ride on the descending phase of these paroxysmal activities. (f) Beta and gamma activities are determined by time-frequency analysis of the paroxysmal ECoG activities recorded from electrode No. 7.
Through reopening of the right frontal craniotomy [
Postoperatively, her consciousness level improved slightly with the disappearance of paroxysmal activities on EEG, but then worsened. Histologically, in the resected cortex, there were numerous senile plaques [
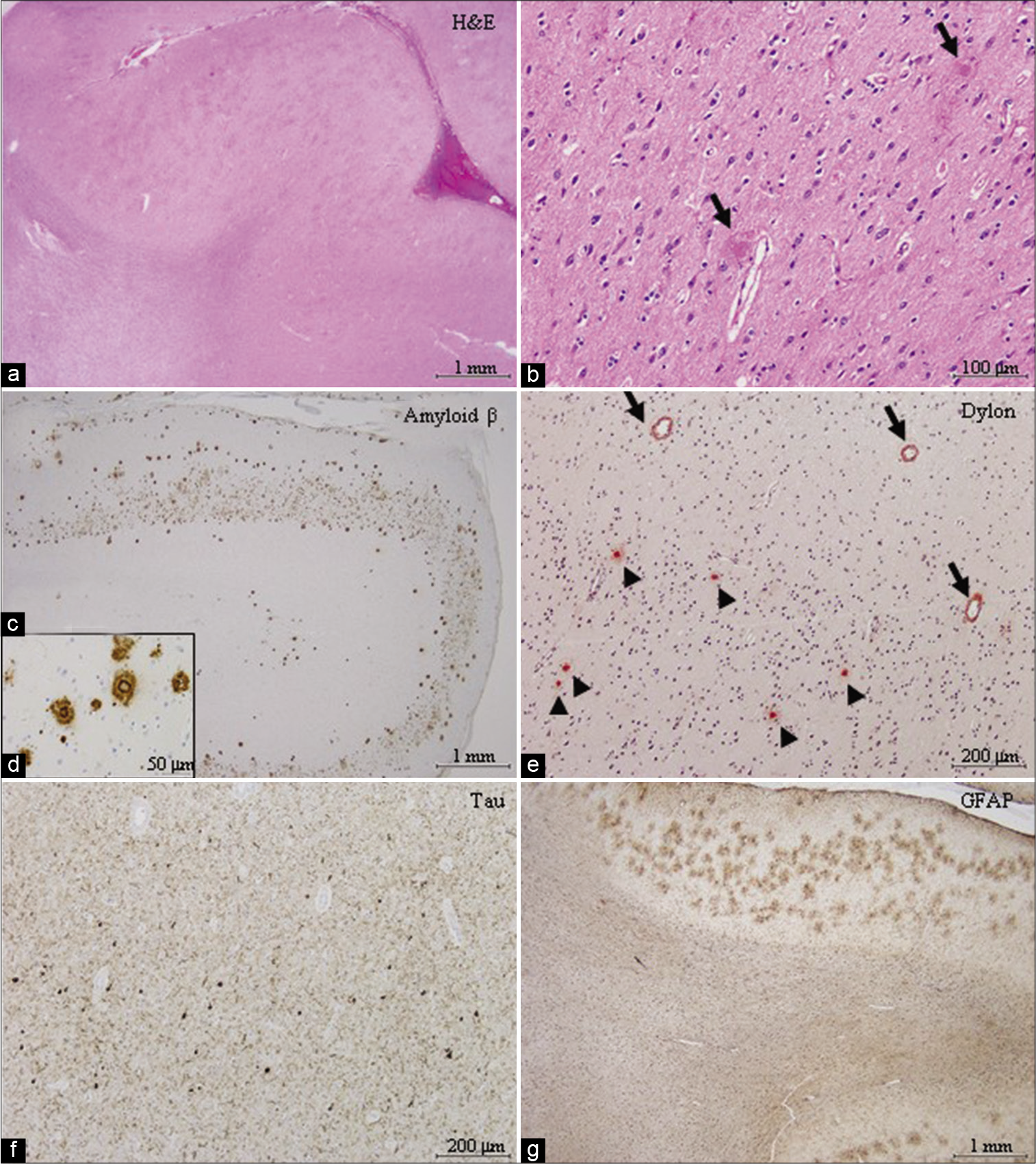
Figure 3:
Histopathological findings of the resected paroxysmal foci. (a and b) Numerous senile plaques associated with the amyloid core (black arrows) are noted in the cortex. Rarefaction of white matter was focally prominent. (c and d) Amyloid β immunopositive senile plaques are located mainly in the cortex. Amyloid β deposition is also observed in some of the meningeal and cortical blood vessels. (e) The amyloid core of the senile plaque tested positive for Dylon staining (arrow heads). Amyloid β deposition was also observed in the cortical blood vessels (arrows). (f) Phosphorylated tau immunopositive neurofibrillary tangles and numerous neuropil threads are observed. (g) Immunostaining for glial fibrillary acidic protein demonstrates severe gliosis in the white matter and prominent astrocytic reaction against senile plaques in the cortex.
DISCUSSION
Retrospectively, in the present case, mild cognitive impairment as the first symptom was not solely attributed to the right frontal meningioma, but mainly to AD. It has been reported that meningioma is the most common incidental neoplastic finding in patients with AD.[
Many seizure semiologies in AD are typical of medial temporal lobe epilepsy (TLE), and interictal paroxysmal activities on routine EEG are commonly detected in the temporal or frontotemporal region.[
In the present case, the focus of the paroxysmal activities was localized in the right frontal region related to the meningioma resection. However, localized epileptogenic lesions, such as tumor invasion or surgical scars, were not histologically verified in the resected cortex. Thus, the exact epileptogenic mechanism of this patient was not straightforward and may have been multifactorial. One possible explanation is that, based on the neurophysiological findings, the presence of the meningioma and its surgery-induced AD-associated epilepsy. Since the development of the EEG abnormality exhibited a temporal relationship with that of hydrocephalus, there is a possibility that hydrocephalus was involved as a worsening factor in the development of NCSE. The fact that ventricular enlargement was more prominent on the ipsilateral side to the EEG abnormality may support the second idea.
It is generally accepted that treatment of epilepsy in AD with selective antiepileptic drugs such as levetiracetam and lamotrigine in low doses is usually well tolerated and efficacious.[
Intraoperative ECoG demonstrated periodic paroxysmal discharges, which had a waveform similar to that of EEG, in the neighboring cortex where the meningioma existed. A notable ECoG finding was that fast-wave activities rode on the descending phase of the major negative component. These fast-wave activities were not recorded on EEG, probably due to the smearing effect.[
Emerging evidence indicates that epilepsy can hasten cognitive decline in patients with AD.[
CONCLUSION
Even when a suspected epileptogenic lesion is encountered, which could cause focal NCSE, AD should be ruled out in elderly patients with progressive cognitive decline.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Born HA. Seizures in Alzheimer’s disease. Neuroscience. 2015. 286: 251-63
2. Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991. 82: 239-59
3. Brugulat-Serrat A, Rojas S, Bargallo N, Conesa G, Minguillon C, Fauria K. Incidental findings on brain MRI of cognitively normal first-degree descendants of patients with Alzheimer’s disease: A cross-sectional analysis from the ALFA (Alzheimer and families) project. BMJ Open. 2017. 7: e013215
4. Cretin B, Sellal F, Philippi N, Bousiges O, Bitonto LD, MartinHunyadi C. Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: Expanding the spectrum of Alzheimer’s disease to an epileptic variant?. J Alzheimers Dis. 2016. 52: 1125-33
5. Hashiguchi K, Morioka T, Yoshida F, Miyagi Y, Nagata S, Sakata A. Correlation between scalp-recorded electroencephalographic and electrocorticographic activities during ictal period. Seizure. 2007. 16: 238-47
6. Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994. 87: 504-10
7. Mukae N, Morioka T, Torio M, Sakata A, Suzuki SO, Iihara K. Continuous ictal discharges with high frequency oscillations confined to the non-sclerotic hippocampus in an epileptic patient with radiation-induced cavernoma in the lateral temporal lobe. Epilepsy Behav Case Rep. 2019. 11: 87-91
8. Sarkis RA, Dickerson BC, Cole AJ, Chemali ZN. Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci. 2016. 28: 56-61
9. Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: A study of temporal lobe resections. Brain. 2016. 139: 2441-55
10. Takenoshita S, Terada S, Yoshida H, Yamaguchi M, Yabe M, Imai N. Validation of Addenbrooke’s cognitive examination III for detecting mild cognitive impairment and dementia in Japan. BMC Geriatr. 2019. 19: 123
11. Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G. Seizures and epileptic activity in the early stages of Alzheimer’s disease. JAMA Neurol. 2013. 70: 1158-66
12. Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017. 16: 311-22
13. Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016. 19: 1085-92
14. Yamamoto K, Tanei Z, Hashimoto T, Wakabayashi T, Okuno H, Naka Y. Chronic optogenetic activation augments aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 2015. 11: 859-65