- Department of Neurosurgery, Desert Regional Medical Center, Palm Springs, California, United States.
- School of Medicine, University of California Riverside, Riverside, California, United States.
- College of Osteopathic Medicine, Kansas City University of Medicine and Biosciences, Kansas City, Missouri, United States.
- Department of Medical Research, University of California Davis, Davis, California, United States.
- Department of Podiatry, Beaumont Hospital, Farmington Hills, Michigan, United States.
- Grossman School of Medicine, New York University, New York, United States.
Correspondence Address:
Brian Fiani
Grossman School of Medicine, New York University, New York, United States.
DOI:10.25259/SNI_596_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brian Fiani1, Ryan Arthur Figueras2, Frank De Stefano3, Neha Gautam4, Asif Khan5, Marisol Soula6. Nonmissile penetrating spinal injuries: Mechanisms, expectations, and management. 25-Nov-2020;11:406
How to cite this URL: Brian Fiani1, Ryan Arthur Figueras2, Frank De Stefano3, Neha Gautam4, Asif Khan5, Marisol Soula6. Nonmissile penetrating spinal injuries: Mechanisms, expectations, and management. 25-Nov-2020;11:406. Available from: https://surgicalneurologyint.com/surgicalint-articles/10411/
Abstract
Background: Nonmissile penetrating spinal injury (NMPSI) is an uncommon form of traumatic injury to the spine. Here, we present a comprehensive and contemporary literature review that provides insight into NMPSI-type injuries, their mechanisms, clinical practice, management, and expectations.
Methods: An extensive review of the published literature was conducted in PubMed, OVID Medline, and EMBASE journals for studies of nonmissile penetrating spine injuries. Terms for search included NMPSI and nonmissile penetrating spinal cord injury. No date restrictions were used.
Results: The search yielded only 17 related articles. Cross-checking of articles was conducted to exclude duplicate articles. The 17 articles were screened for their full text and English language availability. We finalized those articles pertaining to the topic.
Conclusion: The mechanism of injury in NMPSI occurs in two different stages. Immediate injury is caused by direct damage to the neurological structures. The delayed injury response is caused by damage to the spinal vasculature and activated immune response pathways. Computed tomography (CT) angiograms or formal diagnostic angiograms are indicated to identify vascular injury or development of pseudoaneurysm and can be performed both preoperatively and postoperatively. Surgically, decompressive procedures include laminectomies and hemilaminectomies. Dural exploration may be indicated if a cerebrospinal fluid leak with fistula develops from dural puncture. Further research and technologies are being developed to provide patients who have suffered NMPSI with more resources for a better quality of life.
Keywords: Neurotrauma, Nonmissile penetrating spinal injury, Nonmissile, Penetrating spinal injury
INTRODUCTION
Nonmissile penetrating spinal injury (NMPSI) is an uncommon form of traumatic injury to the spine, particularly rare in North America.[
The vast majority of spinal injuries in North America are due to either motor vehicle accidents or falls.[
MECHANISM OF INJURY
Penetrating wounds occur when an object pierces the body in a traumatic fashion that can destroy, disrupt, or contuse the tissue it penetrates and adjacent areas.[
Injuries involving knives usually produce limited injury because knives are classified as low-velocity projectiles. Such low-velocity projectiles will typically cause damage confined to tissues solely within the path of the penetrating object. In contrast, bullet wounds caused by air-powered pellet guns and handguns are classified as medium velocity. These medium-velocity wounds are distinct from low-velocity types in that they cause cavitation of the tissues and extend the area of tissue damage beyond the path of the penetrating object through shock waves.[
Concerning penetrating trauma wounds, the physics of the energy exchange impacts the degree of tissue damage.[
The most commonly occurring NMPSIs are stab wounds of the back directed toward the thoracic spine (up to 63% of cases), the cervical spine (up to 30% of cases), or the lumbar region (6.7% of cases).[
NEUROLOGICAL SEQUELAE
Because NMPSI represents such a small subset of overall spinal injury, guidelines for the management of these patients and the resulting neurological sequelae are elusive. The possible outcomes and neurological deficits observed in patients are vast, ranging from asymptomatic dural tear to injuries of the nerve root, and patients have been observed to experience symptoms from neurapraxia to neurotmesis, with complete SCI being the most detrimental outcome from NMPSI.
Historically, the neurological sequelae for patients with sustained NMPSI are optimistic. According to a 1991 institutional study, a good prognosis has been reported in 50–60% of NMPSI cases, with surgical intervention demonstrating improved neurological function in 7 out of 9 cases.[
The previous studies have shown that 40% of patients with partial spinal cord injuries were able to recover to functional capacity and able to return to previous places of employment, and an additional 57% demonstrated significant recovery, which is defined by the recovery of their physical capacity which allowed them to be gainfully employed.[
INDICATIONS FOR ANGIOGRAM
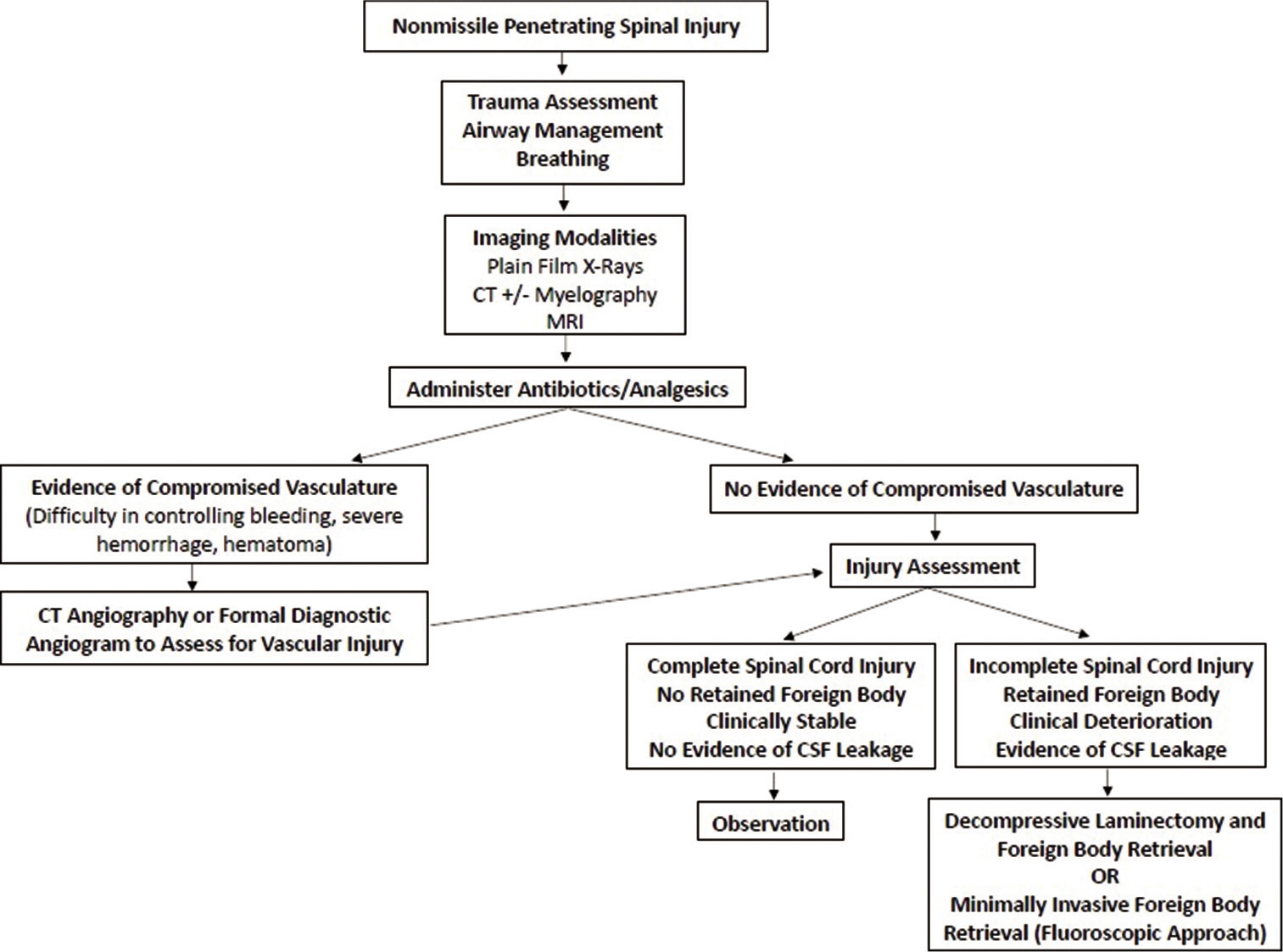
For patients that present with NMPSI, an initial and immediate concern should be the integrity of the vasculature in the spinal region, especially the vertebral arteries. CT angiography has been proven to be a fast, safe, and reliable noninvasive method to assess patients with penetrating neck traumas. It has a specificity of about 100% and sensitivity of 90% for identifying lesions of vascular structures in the neck region.[
Because vascular injuries related to NMPSI are most common in the cervical spine as opposed to thoracic or lumbar spine (comprising up to 40% of penetrating neck traumas), clinicians should observe the patient for signs that are suggestive of vascular injury.[
In some cases of penetrating traumas, such as CSF leaks, a mandatory exploratory surgery is carefully performed to determine the precise injuries of the patient and to surgically correct any concerns such as performing primary dural closure of a CSF leak.[
After open neurovascular surgery or endovascular treatment, there are concerns such as incomplete treatment of vascular injury, delayed cerebral infarcts, or cerebral hemorrhage.[
GENERAL SURGICAL GUIDELINES
The benefit of surgical exploration versus nonsurgical management of NMPSI is a debated topic. Surgical consideration is warranted for progressive neurologic deficits, evidence of RFB, or prolonged CSF leakage.[
Advances in minimally invasive retrieval approaches of a RFB with the use of fluoroscopy have shown promise.[
Standard antibiotic prophylaxis recommendations specifically for NMPSI have not been reported. One report utilized an empiric antibiotic regimen recommended for open fracture protocols. Antibiotics should be given within 4 h of presentation and no later than 48–72 h. Evidence of spinal canal penetration warrants administration of third-generation cephalosporins for central nervous system coverage given concern for meningitis.[
NEW TECHNOLOGY AND RESEARCH FOR RECOVERY
NMPSI has the potential to cause permanent neurological deficits from SCI. In the event of SCI, the communication within the nervous system is disrupted, leading to the loss of essential neurological functions. With technological advancements and growth in innovation, much research focus is being placed on the recovery, or at least improving the functionality, of SCI patients. University of California Los Angeles researchers have created an innovative technique to increase the effective impulse potential through translational perspectives both in the treatment and in the diagnostics of spinal injuries.[
The utilization of electric stimulation is an innovative technique allowing patients with spinal injury to restore limb functionality.[
The amplitude and frequency variations that were provided with the multielectrode positioning also played a crucial role in effectiveness.[
Targeted spinal cord stimulation has shown progress with utilization of neurotechnology to facilitate voluntary control of walking in individuals who sustained a SCI status post-NMPSI.[
CONCLUSION
NMPSI is a devastating traumatic injury that occurs rarely but can have lasting implications. It is important for neurologists and neurosurgeons to have proper understanding of the mechanism of injury, the potential neurological sequelae, and the most recent management guidelines. Workup includes CT angiography or formal diagnostic angiogram either preoperatively or both preoperative and postoperatively. Multielectrode pulse generators that potentiate impulse signals to the spinal cord have shown dramatic advances in NMPSCI patients. However, there is a significant lack of data regarding outcomes and expectations. Likewise, literature on surgical intervention techniques is lacking uniformity and therefore treatment is individualized to each patient. Physician researchers are working on advanced techniques to perfect a standardization of care for NMPSI.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alao T, Waseem M.editors. Neck Trauma. Treasure Island, FL: Stat Pearls; 2020. p.
2. Bledsoe BE, Casey M, Hodnick R. Breaking the surface: Arm yourself with knowledge about penetrating trauma. JEMS. 2012. 37: 58-64
3. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury, Results of the third national acute spinal cord injury randomized controlled trial, National acute spinal cord injury study. JAMA. 1997. 277: 1597-604
4. Enicker B, Gonya S, Hardcastle TC. Spinal stab injury with retained knife blades: 51 Consecutive patients managed at a regional referral unit. Injury. 2015. 46: 1726-33
5. Goldberg AL, Kershah SM. Advances in imaging of vertebral and spinal cord injury. J Spinal Cord Med. 2010. 33: 105-16
6. Goyal RS, Goyal NK, Salunke P. Non-missile penetrating spinal injuries. Indian J Neurotrauma. 2009. 6: 81-4
7. Groen RJ, Kafiluddin EA, Hamburger HL, Veldhuizen EJ. Spinal cord injury with a stingray spine. Acta Neurochir (Wien). 2002. 144: 507-8
8. Kramer MM, Acker A, Ohana N.editors. Penetrating spinal cord injury. Essentials of Spinal Cord Injury Medicine. London: Intech Open; 2018. p.
9. Kuhajda I, Zarogoulidis K, Kougioumtzi I, Huang H, Li Q, Dryllis G. Penetrating trauma. J Thorac Dis. 2014. 6: S461-5
10. Kumar A, Pandey PN, Ghani A, Jaiswal G. Penetrating spinal injuries and their management. J Craniovertebr Junction Spine. 2011. 2: 57-61
11. Lefebvre CW, Babich JP, Grendell JH, Grendell JH, Heffner JE, Thibault R.editors. Penetrating wounds. Encyclopedia of Intensive Care Medicine. Berlin, New York: Springer; 2012. p. 1699-703
12. Levy ML, Gans W, Wijesinghe HS, SooHoo WE, Adkins RH, Stillerman CB. Use of methylprednisolone as an adjunct in the management of patients with penetrating spinal cord injury: Outcome analysis. Neurosurgery. 1996. 39: 1141-9
13. Lipschitz R, Block J. Stab wounds of the spinal cord. Lancet. 1962. 280: 169-72
14. Martin MDWolfla CE. Penetrating Spine Trauma, Neupsy Key. Available from: https://www.neupsykey.com/penetrating-spine-trauma [Last accessed on 2020 Jul 29].
15. Moldovan K, Telfeian AE, Fridley JS, Gokaslan ZL, Aghion D, Oyelese AA. Minimally invasive approach to non-missile penetrating spinal injury with resultant retained foreign body: A case report and review of the literature. Clin Neurol Neurosurg. 2019. 184: 105405
16. Nasr A, de Oliveira JT, Mazepa MM, de Albuquerque CL, Martini GS, Nazario M. Evaluation of the use of tomography in penetrating neck trauma. Rev Col Bras Cir. 2015. 42: 215-9
17. Peacock WJ, Shrosbree RD, Key AG. A review of 450 stabwounds of the spinal cord. S Afr Med J. 1977. 51: 961-4
18. Peng CW, Chou BT, Bendo JA, Spivak JM. Vertebral artery injury in cervical spine surgery: Anatomical considerations, management, and preventive measures. Spine J. 2009. 9: 70-6
19. Ramani D. Spinal Injuries: A New Technology of Electrostimulation for a More Effective Approach, United States: American Association for the Advancement of Science. Available from: https://www.eurekalert.org/pub_releases/2019-11/sisd-sia111319.php [Last accessed on 2020 Jul 29].
20. Shahlaie K, Chang DJ, Anderson JT. Nonmissile penetrating spinal injury, Case report and review of the literature. J Neurosurg Spine. 2006. 4: 400-8
21. Simpson RK, Venger BH, Narayan RK. Treatment of acute penetrating injuries of the spine: A retrospective analysis. J Trauma. 1989. 29: 42-6
22. Smith C, White JB. Penetrating knife injuries to the spine: Management considerations and literature review. Interdiscip Neurosurg. 2014. 1: 3-4
23. Sperry JL, Moore EE, Coimbra R, Croce M, Davis JW, Karmy-Jones R. Western trauma association critical decisions in trauma. J Trauma Acute Care Surg. 2013. 75: 936-40
24. Steenburg SD, Sliker CW, Shanmuganathan K, Siegel EL. Imaging evaluation of penetrating neck injuries. Radiographics. 2010. 30: 869-86
25. Takhtani D, Melhem ER. MR imaging in cervical spine trauma. Clin Sports Med. 2002. 21: 49-75
26. Thakur RC, Khosla VK, Kak VK. Non-missile penetrating injuries of the spine. Acta Neurochir (Wien). 1991. 113: 144-8
27. Villarreal-García FI, Reyes-Fernández PM, MartínezGutiérrez OA, Peña-Martínez VM, Morales-Ávalos R. Direct withdrawal of a knife in the lumbar spinal canal in a patient without neurological deficit: Case report and review of the literature. Spinal Cord Ser Cases. 2018. 4: 48
28. Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018. 563: 65-71
29. Wallace DJ, Sy C, Peitz G, Grandhi R. Management of non-missile penetrating spinal injury. Neurosurg Rev. 2019. 42: 791-8
30. Xia X, Zhang F, Lu F, Jiang J, Wang L, Ma X. Stab wound with lodged knife tip causing spinal cord and vertebral artery injuries: Case report and literature review. Spine (Phila Pa 1976). 2012. 37: E931-4
31. Yoneoka Y, Akiyama K, Seki Y. Glass fragment injury to the craniocervical junction with interatlantooccipital penetration to the subarachnoid space: Not-to-be-missed important aspects of craniocervical trauma even in the middle of the COVID-19 pandemic: Case report and review of literature. World Neurosurg. 2020. 141: 402-5
32. Yoon J, Efendy J, Szkandera B, Redmond M. Non missile penetrating spinal injury. J Clin Neurosci. 2019. 67: 239-43