- Department of Neurosciences, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- Department of Anaesthesiology and Intensive Care, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Malaysia.
Correspondence Address:
Mohamad Hasyizan Hassan, Department of Anaesthesiology and Intensive Care, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Malaysia.
DOI:10.25259/SNI_526_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naavin Kumar Balakrishnan1, Abdul Rahman Izaini Ghani1, Mohamad Hasyizan Hassan2, Laila Ab. Mukmin2, W. Mohd Nazaruddin W. Hassan2. Nosocomial Elizabethkingia meningoseptica meningitis and bacteremia in a post transsphenoidal hypophysectomy complicated with sagittal sinus thrombosis: A case report. 19-Aug-2022;13:370
How to cite this URL: Naavin Kumar Balakrishnan1, Abdul Rahman Izaini Ghani1, Mohamad Hasyizan Hassan2, Laila Ab. Mukmin2, W. Mohd Nazaruddin W. Hassan2. Nosocomial Elizabethkingia meningoseptica meningitis and bacteremia in a post transsphenoidal hypophysectomy complicated with sagittal sinus thrombosis: A case report. 19-Aug-2022;13:370. Available from: https://surgicalneurologyint.com/surgicalint-articles/11806/
Abstract
Background: Elizabethkingia meningoseptica meningitis is rare and challenging to manage infection. As this infection is always associated with superimposed multidrug-resistant organisms, a combination and prolonged antibiotic treatment are necessary to ensure the complete eradication of infections.
Case Description: We report successful antibiotic therapies in a patient with E. meningoseptica bacteremia and meningitis complicated with superimposed extreme-drug-resistant Acinetobacter baumannii infection in a patient post transsphenoidal hypophysectomy complicated with central venous thrombosis.
Conclusion: Antibiotic combination therapy with prolonged duration in those with E. meningoseptica with concomitant multi-resistant organisms is needed. Diagnosing associated prothrombotic risk with the infection and prompt treatment would also be essential.
Keywords: Elizabethkingia meningoseptica, Intensive care, Multidrug resistance, Neurosurgery, Sinus thrombosis
INTRODUCTION
Elizabethkingia spp. can be divided into six subtypes, and Elizabethkingia meningoseptica is one of them. Previously, Elizabethkingia species was subdivided under the Chryseobacterium genus. It is a Gram-negative bacillus with a characteristic of oxidase positive, nonmotile, nonfermentative, and nonsugar fermenting.[
CASE REPORT
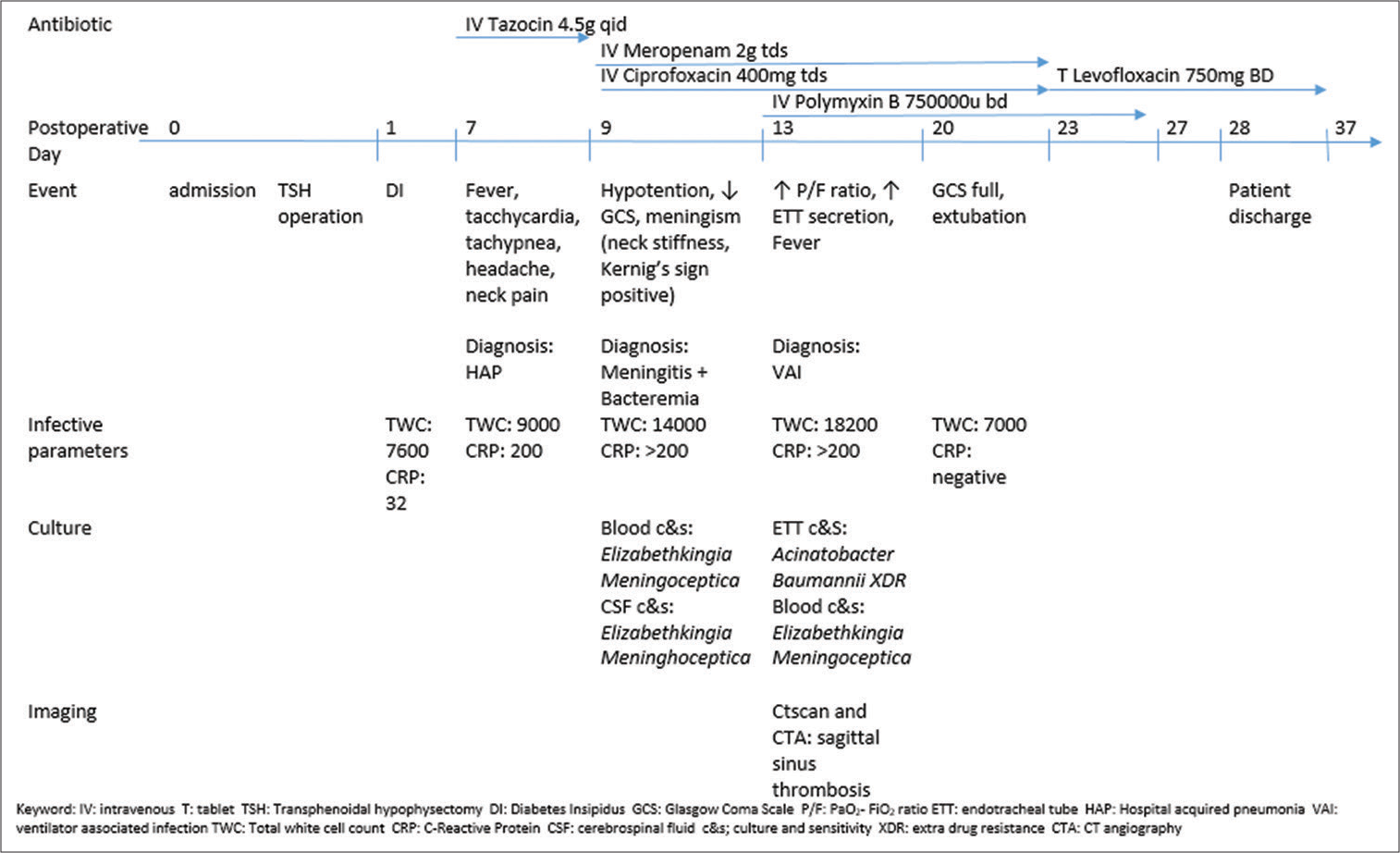
A 65-year-old gentleman with underlying allergic rhinitis, eczema, dyslipidemia, and benign prostatic hyperplasia initially presented with insidious onset of the left eye blurring of vision over the past 3 years which progressively worsened. On examination, the patient had left visual field quadrantanopia with no papilloedema and no other neurological deficit. MRI brain reported a pituitary macroadenoma size 2.0 cm × 1.8 cm × 2.0 with compression to the left optic chiasma. The patient’s neuroendocrine workup showed low cortisol, low growth hormone, and low testosterone. Other neuroendocrine hormones were normal. He was electively admitted for TSH and excision of the tumor. Intraoperatively, the dura was not breached, and the procedure went uneventful. He was extubated postoperatively and transferred to the ward for observation. He developed polyuria on the 1st day postoperatively and was treated for cranial diabetes insipidus with desmopressin. On day 7, he was febrile, tachypneic, tachycardic, and had minimal cough with no neurological symptoms. There was no cerebrospinal fluid (CSF) rhinorrhea on examination. Air entry in his lung was equal with no adventitious sound. The chest X-ray revealed minimal perihilar haziness. White blood cell (WBC) count was 9000 cells/cm3, and C-reactive protein was 200 mg/L, and he was started on IV Tazosin 4.5 g 6 hourly empirically for hospital acquired pneumonia. Subsequently, the patient complained of headache and neck pain on the same day. After counseling, he was indecisive about a lumbar puncture to diagnose meningitis. On day 9, the patient’s Glasgow Coma Scale dropped from E4V5M6 to E3V3M5. The pupils were still 3/3 reactive but with neck stiffness with positive Kernig’s and Brudzenski signs. Blood sepsis parameter showed increased WBC to 14,000 cells/cm3 and CRP >200 mg/L. Blood culture preliminary report taken on day 7 of illness came back as E. meningoseptica, Carbapenam Resistance Enterobacteriaceae (CRE). On MacConkey agar, a nonlactose fermenter colony was seen, and the Gram stain showed the Gram-negative bacilli and nonmotile, and oxidative positive was identified by Vitek GN ID card. Antibiotic susceptibility testing using minimum inhibitory concentration and not disk diffusion following Clinical and Laboratory Standard Institute, CLSI M100. The patient was intubated for septic shock with meningitis and transferred to the neurocritical care unit for intensive care management. He was started on IV Meropenem 2 g TDS and IV Ciprofloxacin 400 mg TDS. On day 9, lumbar puncture and the CSF results were reported as clear and yellowish, total WBC increased 10 cells/mm3 with predominant polymorphs cell, RBC was nil glucose 2.2 mmol/L, and protein 0.57 g/L with positive culture E. meningoseptica CRE. CSF lactate dehydrogenase was not performed. The patient was diagnosed with bacteremia and meningitis due to positive clinical signs and multiple isolations of E. meningoseptica from the blood and CSF cultures and biochemistry profiles. Subsequently, the patient did not improve neurologically post antibiotics despite improvement of the septic parameters and was subjected to a contrast-enhanced CT brain. On assessment of the CT scan, the patient developed short-segment superior sagittal sinus thrombosis. The patient was started on SC clexane 80 mg BD. On day 13, the tracheal aspirate culture and sensitivity grew Acinetobacter baumannii Extreme Drug-Resistant (XDR). IV Polymyxin B 750000 U BD was added for this patient to cover the CRE and XDR. The patient completed IV Polymyxin for 14 days and IV Meropenem for 14 days. IV Ciprofloxacin was completed for 14 days and followed by 14 days of T. Levofloxacin 750 mg BD. The patient’s condition markedly improved, and he was extubated well on day 17. The course of culture and sensitivity and antibiotics therapy is simplified in
Figure 1:
Clinical course of symptoms, culture, and antibiotic therapy for the patient. IV: Intravenous, T: tablet, TSH: transphenoidal hypophysectomy, DI: diabetes insipidus, GCS: Glasgow Coma Scale, P/F: PaO2-FiO2 ratio, ETT: endotracheal tube, HAP: hospital-acquired pneumonia, VAI: ventilator-associated infection, TWC: total white cell count, CRP: C-reactive protein, CSF: cerebrospinal fluid, c&s: culture and sensitivity, XDR: extra drug resistance, CTA: CT angiography.
DISCUSSION
Elizabethkingia species are oxidase-positive, nonmotile, nonfermentative, and nonglucose fermenting Gram-negative aerobic bacilli, and E. meningoseptica is one of the medically important species.[
Elizabethkingia spp. is an emerging nosocomial pathogen. It is known that E. meningoseptica colonized the human oropharynx, respiratory secretions, aerosol tubes, endotracheal tubes, and the respiratory tract in ventilated adult patients.[
A possible explanation for the high mortality rate may be its potential to form biofilms. The biofilms produced by biofilm-forming pathogens, like Elizabethkingia spp., might decrease the immune response and increase resistance against antibiotics, resulting in a high mortality rate. In addition, prolonged antibiotic treatment regimens are necessary to eradicate the pathogen successfully. Moreover, it is known that Elizabethkingia spp. has virulence factors, including intracellular invasion and chromosomal and plasmid-mediated resistance to many antimicrobial drugs.[
Our patient’s recognizable risk factors for E. meningoseptica are underlying hypocortisolism and prolonged intensive care and hospital stay. Furthermore, the superimposed multi-resistant organism with Elizabethkingia is common, making it more complicated for antibiotic management. Judicious use of antibiotic combination therapy with the frequent assessment of the patient clinical condition is necessary. The antibiotic of choice must have good blood barrier penetration in patients with meningitis. In our patient, since the patient also contracted XDR infection, IV Polymyxin B was also started in an antibiotic combination. Prolonged antibiotic therapy is also necessary due to the formation of biofilm. That is why we continued with oral Levofloxacin after the completion of IV ciprofloxacin. Although the mortality rate with Elizabethkingia is reported to be more than 50%, frequent assessment and initiation of correct antibiotic regimes may improve the patient’s outcome. Moreover, since one of the virulence factor genes in Elizabethkingia contains phospholipase C, we postulate that the incidence of central venous thrombosis in this patient might also be contributed by the prothrombotic property of this infection.[
CONCLUSION
Nosocomial E. meningoseptica is rare but difficult to manage and often requires antibiotic combination therapy with prolonged duration, especially in those who have superimposed multi-resistant organisms. Incidence of central venous thrombosis might also be contributed by the infection necessitating prompt therapy of antithrombotic agents and optimization of intravascular volume.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Balm MN, Salmon S, Jureen R, Teo C, Mahdi R, Seetoh T. Bad design, bad practices, bad bugs: Frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J Hosp Infect. 2013. 85: 134-40
2. Ceyhan M, Yildirim I, Tekeli A, Yurdakok M, Us E, Altun B, Kutluk T, Cengiz AB. A Chryseobacterium meningosepticum outbreak observed in 3 clusters involving both neonatal and non-neonatal pediatric patients. Am J Infect Control. 2008. 36: 453-7
3. González LJ, Vila AJ. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob Agents Chemother. 2012. 56: 1686-92
4. Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica An important emerging pathogen causing healthcare-associated infections. J Hosp Infect. 2014. 86: 244-9
5. Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005. 55: 1287-93
6. Lin PY, Chiu CH, Chu C, Tang P, Su LH. Invasion of murine respiratory tract epithelial cells by Chryseobacterium meningosepticum and identification of genes present specifically in an invasive strain. New Microbiol. 2006. 29: 55-62
7. Maraki S, Scoulica E, Manoura A, Papageorgiou N, Giannakopoulou C, Galanakis E. A Chryseobacterium meningosepticum colonization outbreak in a neonatal intensive care unit. Eur J Clin Microbiol Infect Dis. 2009. 28: 1415-9
8. Ramanan P, Razonable RR. Elizabethkingia species sepsis after lung transplantation: Case report and literature review. Transpl Infect Dis. 2013. 15: E229-34
9. Tak V, Mathur P, Varghese P, Misra MC. Elizabethkingia meningoseptica An emerging pathogen causing meningitis in a hospitalized adult trauma patient. Indian J Med Microbiol. 2013. 31: 293-5
10. Zajmi A, Teo J, Yeo CC. Epidemiology and characteristics of Elizabethkingia spp. Infections in Southeast Asia. Microorganisms. 2022. 10: 882