- NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_276_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein, Renee D. Hollingsworth. Nursing review of diagnosis and treatment of lumbar degenerative spondylolisthesis. 10-Oct-2017;8:246
How to cite this URL: Nancy E. Epstein, Renee D. Hollingsworth. Nursing review of diagnosis and treatment of lumbar degenerative spondylolisthesis. 10-Oct-2017;8:246. Available from: http://surgicalneurologyint.com/surgicalint-articles/nursing-review-of-diagnosis-and-treatment-of-lumbar-degenerative-spondylolisthesis/
Abstract
Background:In the lumbar spine, degenerative spondylolisthesis or degenerative (not traumatic) slippage of one vertebral body over another is divided into 4 grades – grade I (25%), grade II (50%), grade III (75%), and grade IV (100%). Dynamic X-rays, magnetic resonance (MR), and computed tomography (CT) scans document the slip secondary to arthritic changes of the facet joint plus stenosis, ossification of the yellow ligament, disc herniations, and synovial cysts. MR best demonstrates soft tissue pathology whereas CT better delineates ossific/calcified disease.
Methods:Grade I degenerative spondylolisthesis, typically found at the L4–L5 level followed by L3–L4 and L5S1, is more common in females (ratio 2:1) over the age of 65. Symptoms include radiculopathy (root pain) and neurogenic claudication (e.g., pain with ambulation, requiring the patient to stop, rest, sit down). Symptoms/signs may include unilateral/bilateral radiculopathy and uni/multifocal motor, reflex, and sensory deficits in. Some may also present with a cauda equina syndrome (e.g., paraparesis/sphincter dysfunction).
Results:Surgery for grade I-II spondylolisthesis may include laminectomy alone, laminectomy/noninstrumented fusion or with an instrumented fusion. Older patients with osteoporosis are more likely to have no fusion or a noninstrumented fusion. All fusions utilize autograft harvested from the laminectomy that may or may not be combined with a bone graft expander (to increase the fusion mass) combined with autogenous bone marrow aspirate. The fusion mass is placed over the transverse processes following decortication.
Conclusions:Patients with multilevel spinal stenosis and degenerative spondylolisthesis may require decompressive lumbar laminectomies alone or in combination with noninstrumented or instrumented fusions.
Keywords: Degenerative spondylolisthesis, instrumented fusion, laminectomy alone, lumbar stenosis, neurogenic claudication, noninstrumented fusion, spinal stenosis, radiculopathy
INTRODUCTION
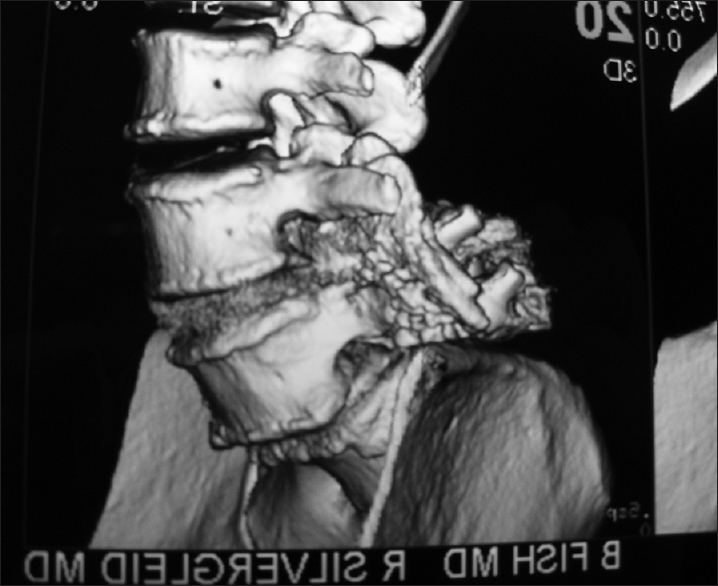
In the lumbar spine, degenerative spondylolisthesis (DS) is most commonly attributed to degenerative changes of the facet joints at the L4–L5, followed by the L3–L4 and L5S1 levels [Figures
Figure 2
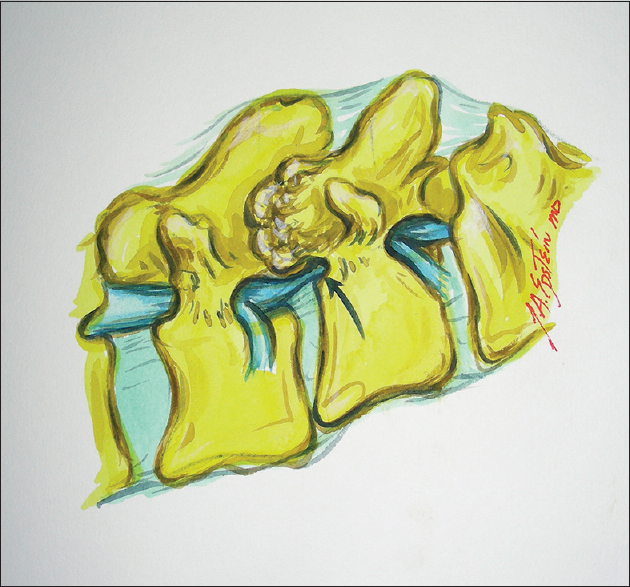
With lumbar stenosis and degenerative spondylolisthesis, there is a marked increase in lateral and foraminal stenosis at the level of the slip. Note in this illustration (Joseph A. Epstein M.D., copyright Nancy E. Epstein M.D.) there is greater right lateral/foraminal compromise attributed to greater hypertrophic changes of the right facet joint and some extrusion of the degenerated synovial cyst into the right L4–L5 foramen compressing both L5 roots (seen here) and also foraminally/superiorly the L4 root
Clinical and neurological presentation for patients with degenerative spondylolisthesis
Grade I DS, largely attributed to the degenerative changes of the facet joints, is most typically found in females vs. males (2–4:1) over the age of 65. Patients may present with unilateral or bilateral radiculopathy (symptoms of root compression) involving the femoral (L2–L4) or sciatic nerve (L5–S1) distributions.
Femoral nerve pathology (L2–L4 roots)
Femoral nerve pathology between L1–L4 levels (L2, L3, L4 roots at the L1/L2, L2/L3, L3/L4 levels) may result in compression of the thecal sac and individual nerve roots. This may contribute to proximal iliopsoas/quadriceps weakness accompanied by loss of the Patellar response, as well as decreased pin appreciation in the L2–L4 distributions (over the thigh and medial calf).
Sciatic nerve root pathology L5S1
Sciatic nerve pathology at the L4–L5 or L5S1 levels compresses the thecal sac and the L5 and/or S1 nerve roots. Patients present with “sciatica” associated with a unilateral or bilateral foot drop (extensor hallucis longus: L5) and/or plantar flexor weakness (S1), a loss of the Achilles response, and decreased pin appreciation in the L5 (lateral calf/dorsum of foot) or S1 distributions (lateral/bottom of the foot).
X-ray findings for degenerative spondylolisthesis
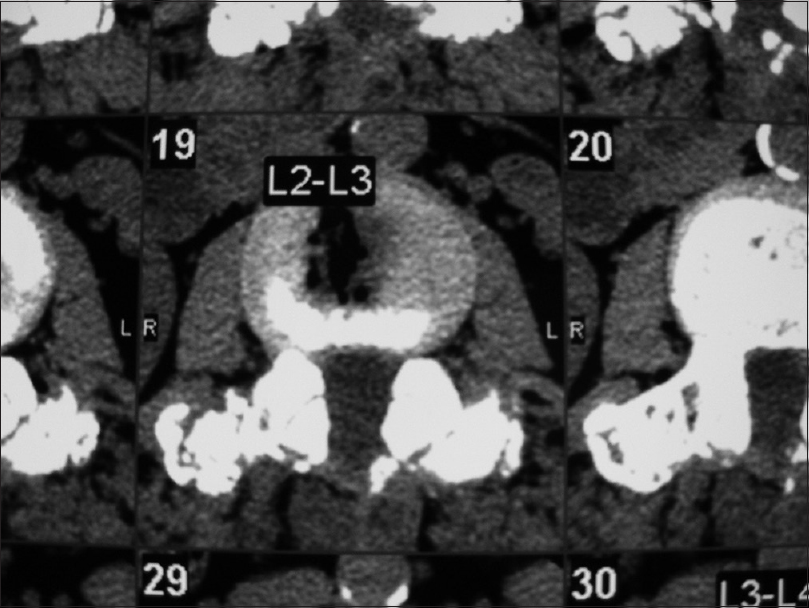
Plain X-ray of the lumbar spine include anterior/posterior films (AP), and flexion/extension studies; the latter look for motion/active slippage. On the AP images, one can readily see alignment, which may be straight, or there may be a curvature indicating instability (e.g., scoliosis). On the lateral plain X-ray, the vertebral bodies should be aligned one on top of the other. However, for DS, there may be an olisthy (slippage). For DS, this most commonly occurs at the L4–L5 level, followed in frequency by the L3–L4, L5–S1, and L2–L3 levels. The slip occurs secondary to the degenerative changes of the facet joints. The extent of the slip is graded I–IV: most patients have a grade I slip (25% of the vertebral body width) followed by a grade II slip (50%). The width of the lumbar canal can be directly measured on these films from the mid aspect of the vertebral body in front (anteriorly) to the dorsal (back) interlaminar line (line created by the lamina coming together to form the spinous process). The normal canal width should be 20 mm. If it is smaller, this is considered spinal stenosis; congenital stenosis 10–12 mm, relative (acquired stenosis) >13 mm.
Lumbar neuroanatomy
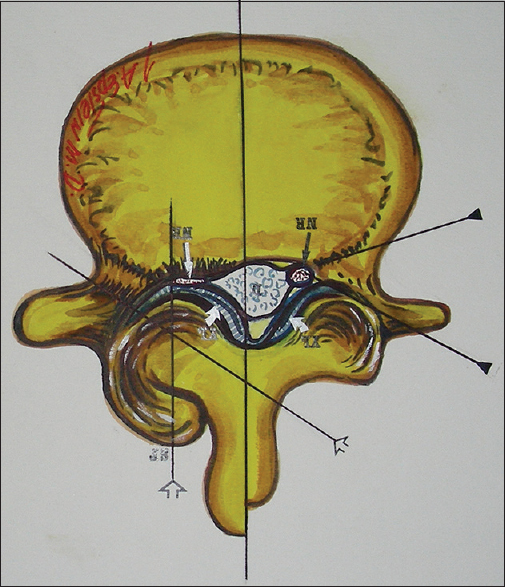
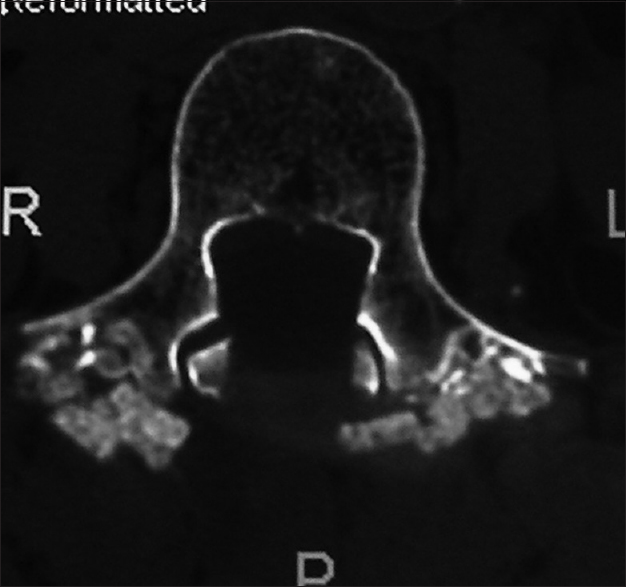
In the lumbar spine, the spinal cord ends at the T12–L1 level; below this you have the cauda equina or a collection of nerve roots. At each spinal level, nerve roots exit the spinal canal. For example, at the L4–L5 level, the L5 roots travel inferiorly into the lateral recesses; superiorly and foraminally the L4 nerve root exits as well. Root compression may be attributed to spinal stenosis alone; DS with the focal slippage further narrowed the front/back diameter of the canal (AP diameter) [Figures
Radiographic findings for degenerative spondylolisthesis
Magnetic resonance findings for degenerative spondylolisthesis
MR studies are best at demonstrating soft tissue changes in the spinal canal. These studies actually do not define bone; rather, what you see is the water or fat content in the bone marrow. Neural tissues, fat, synovial cysts, hypertrophied ligament, and other noncalcified structures are more readily seen on MR studies in three dimensions; coronal (frontal/AP view), lateral (to the side, sagittal), and in cross-section (axial views). T1 MR studies show the spinal fluid as dark (hypointense), whereas T2-weighted images make the spinal fluid white (hyperintense; e.g., the “myelographic image”). When dye is administered to a T1-weighted MR study consisting of gadolinium DTPA (not iodine based), tumors and other structures (e.g., areas of infection, vascular malformations, other) with increased vascularity may “light up” (enhance).
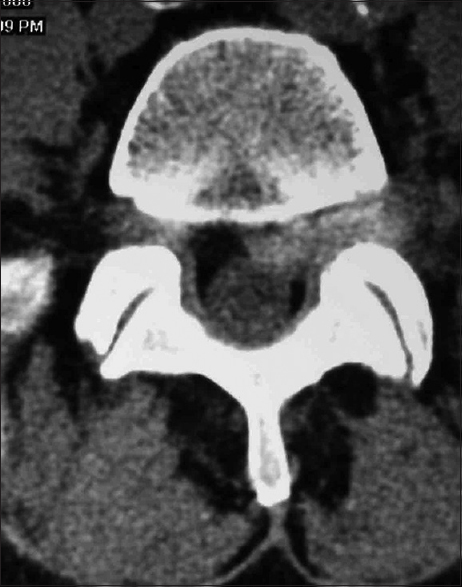
Computed tomography findings for degenerative spondylolisthesis
CT scans for DS are better at demonstrating ossification/calcification changes. These studies provide a positive image of bone that appears hyperdense or white; therefore, they better define the actual extent of bony stenosis. Furthermore, with DS, they confirm the degenerative slip and one can also rule out a fracture (e.g., lysis defect: fracture across the pars interarticularis). OYL seen most commonly with DS appears hyperdense (white) on CT images if it is very calcified. In addition, synovial cysts and even discs (limbus fractures) that are calcified may also appear hyperdense of CT studies.
Magnetic resonance/computed tomography findings for ossification of the yellow ligament with degenerative spondylolisthesis
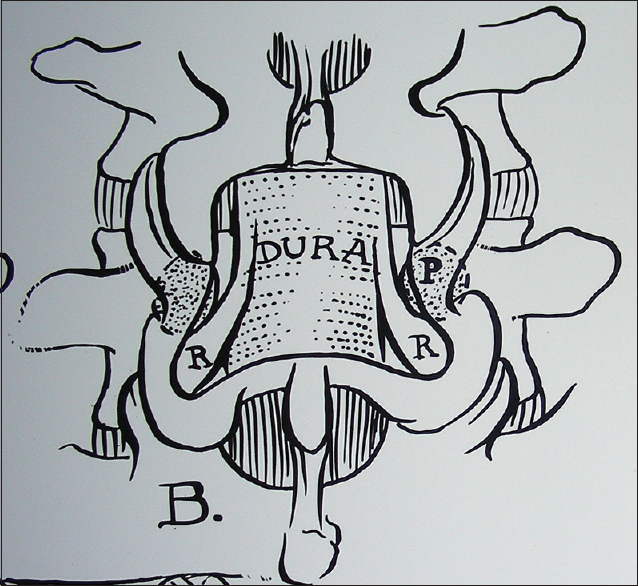
OYL is characterized by hypertrophy/ossific changes of this ligament (ligamentum flavum). The ligament arises bilaterally/foraminally ventral to the capsular ligaments bilaterally. It is also affixed to the superior margin of each lamina (caudal) and extends superiorly to the mid aspect of each cephalad lamina. It only appears to “cross” the midline when extensive degenerative changes have occurred. With DS, the superior lamina contributes to maximal AP diameter thecal sac compression. Next, however, is marked lateral recess compromise (root compression) attributed to hypertrophy/ossification of the yellow ligament.
Magnetic resonance/computed tomography findings for synovial cysts with degenerative spondylolisthesis
At all levels of the spinal canal, the facet joints contain synovial capsules. However, in the lumbar spine, these synovial tissues may herniate into the spinal canal. This is largely attributed to disruption of the facet joints most commonly seen at the L4–L5 level, followed by the L3–L4, L5–S1, and L2–L3 levels. These may appear unilaterally or bilaterally, and are often seen in conjunction with DS. On MR, they appear hyperintense on T2-weighted studies (e.g., internal crankcase fluid content of the cyst). However, the remaining capsule, which is often thick, tenacious, and much larger than the actual “cystic component,” may appear isointense on T1 and T2 studies or indeed become hypointense if calcified/ossified. Notably, synovial cysts not only extend inferiorly often markedly compressing the entire thecal/dural sac but also fill the lateral recesses and compress the inferiorly exiting nerve roots. Furthermore, they extend superiorly and foraminally where they also compromise the superiorly/far laterally exiting nerve root. This would mean at the L4–L5 level, the thecal sac plus L5 and L4 nerve roots may be compromised unilaterally or bilaterally.
Magnetic resonance/computed tomography findings for disc herniations with degenerative spondylolisthesis
DS is rarely associated with a disc herniation. Mostly, on MR and CT, there is a “pseudodisc” noted anteriorly that results from the slip. At surgery, discectomy for a “pseudodisc” should be avoided as a laminectomy alone often provides sufficient dorsolateral decompression, and removal of the “pseduodisc” will only further destabilize the level of the DS slip. If an extruded or sequestrated disc is present (e.g., disc spread beyond the confines of the annulus), a noninstrumented or on occasion an instrumented fusion may become warranted. It is often critical on the preoperative studies to check the CT carefully to determine whether the arthrotic L4–L5 facets joints have already spontaneously fused. If this were the case, there would be no reason to perform any fusion.
SURGERY FOR DEGENERATIVE SPONDYLOLISTHESIS
Prior studies documented treating spinal stenosis/degenerative spondylolisthesis with laminectomy alone
There are multiple prior studies in the literature documenting how stenosis/DS may be successfully treated with laminectomy alone [Figures
Commentary on two 2016 studies regarding treatment of stenosis/degenerative spondylolisthesis
In 2016, Epstein reviewed (e.g., in commentaries) two articles from the New England Journal of Medicine that came to opposite conclusions regarding the treatment of lumbar stenosis/DS: without vs. with fusion.[
Decompressive laminectomy for degenerative spondylolisthesis
Patients with a grade I spondylolisthesis (25% slippage) may be managed with laminectomy alone, laminectomy with a noninstrumented fusion, and occasionally with an instrumented fusion [Figures
Lumbar laminectomy, performed through a midline vertical incision in the lower back, allows for removal of select spinous processes and laminae. The muscle/soft tissue is dissected away from the central bony structures (laminae and spinous processes), and a retractor is placed (we use a SuperSlide) with large cottonoids applied to the skin edges over the muscle/adipose tissue. Using a rib cutter, the spinous processes are removed; if any fusion is anticipated, dry cottonoids may collect bone marrow aspirate (BMA) or back bleeding from the spinous process. Any harvested bone during the laminectomy may be placed in the BMA after soft tissue has been removed. Additionally, the autologous blood squeezed out of the soaked cottonoids and may be applied later if a bone graft fillers is needed to supplement the bony fusion mass.
The microscope may then be brought into the operative field and utilized for the duration of the surgery. Under the operating microscope, an up-biting curette is used to dissect the ligamentum flavum away from the superior lamina (typically L5 at the L5S1 level). Next, a cottonoid is placed over the dura, allowing a Kerrison rongeur to be used to remove lamina. If no fusion is to be performed, the bone does not have to be saved, and a diamond drill (usually Midas Rex with Am 33 diamond bit) may be used to thin down/remove a majority of the laminae. The Kerrisons are then used to remove the laminae; 2-3-4 mm Rongeurs. If OYL is severe and compression is marked with complex pathology, one can always start with the decompression at a less severely compromised level. This will help avoid a cerebrospinal fluid fistula, as then one can work cephalad/caudad to very slowly and meticulously dissect/decompress the most pathologically compressed level (e.g., maximal stenosis/DS). Laminectomies may cover 2, 3, 4, 5, or up to all 6 levels (e.g. L4–L5, L3–L5, L3–S1, L2–S1, up to L1–S1 or other variants).
Degenerative spondylolisthesis with foraminal/far lateral pathology: Potential indications for fusions
For patients with DS, additional foraminal/far lateral pathology at the level of the slip increases the necessity to consider a noninstrumented or instrumented fusion. Such pathology may include extensive foraminal/far lateral OYL, synovial cysts, and disc herniations/limbus fractures. On preoperative CT studies (check coronal and parasagittal bone window studies), some patients will exhibit spontaneous traction spurs crossing the level of the DS, and/or fusion of the vertebral bodies or of the facets themselves making additional fusion unnecessary. For those with synovial cysts, which almost always extend superiorly/foraminally/far laterally, decompression and partial or in some cases complete facet resection will be warranted. This is also true for foraminal and/or far lateral discs where partial/total facet resection has been performed, noninstrumented or instrumented fusions may be required.
Indications for noninstrumented lumbar fusions for degenerative spondylolisthesis
Particularly in older patients with osteoporosis and grade I DS at one or two levels (e.g., typically L4–L5 followed by L3–L4), a noninstrumented or in-situ fusion may be warranted. Typically, preoperative MR, CT, and dynamic films confirm mild/moderate spondylolisthesis (e.g., often without active motion); intraoperatively, clamps may also be placed on the spinous processes of the levels involved to determine the amount of motion under anesthesia. If the decision is finalized to perform a noninstrumented fusion, muscle is dissected laterally to create a pocket over the skeletonized transverse processes (TP); the pocket should be big enough to later receive the lamina autograft/and bone void filler (if used). For example at the L4–L5 level, the L5 TP is exposed; at the L3–L4 level, the L4 TP is exposed; at the L23 level the L3 TP is exposed. Next, BMA (usually 10 cc for a 10 cm × 2.5 cm strip) is applied over a bone graft expander (e.g., we use Nanoss, Regeneration Technologies Inc., Alachua, Fl, USA, a product that traps the stem cells; there are also multiple other products). As previously noted, BMA is harvested with dry cottonoids as soon as the rib cutter is used to remove the spinous processes; blood/BMA is collected by “squeezing” out the cottonoids into a separate sterile jar. Once laminectomy is complete, and after decorticating the transverse processes, the autograft bone harvested during the decompression is first applied over the TP followed by dorsal application of half of the bone graft expander strips to either side.
Indications for instrumented lumbar fusions for degenerative spondylolisthesis
For younger patients with instability/DS/lysis (fracture of the pars) documented on dynamic X-rays (flexion/extension studies) or with a unilateral (one sided) or bilateral (both sided) full facetectomy (total removal of the facet joint) an instrumented fusion may be warranted. This typically requires the application of screws into the pedicles on both sides of the spinal canal under electromyographic monitoring with fluoroscopic control. When instrumented fusions are performed, bone graft and bone graft expanders (Nanoss) are similarly applied bilaterally over the transverse processes just as was done with the noninstrumented fusion. Here, the instrumentation may increase the perioperative risks to neural/vascular structures, whereas postoperatively the infection risk and potential for reoperation to remove failed instrumentation must be considered.
CONCLUSIONS
The vast majority of patients presenting with symptomatic spinal stenosis and DS have grade I spondylolisthesis (25% slippage/olisthy) or grade II olisthesis (50%). The optimal surgical management is a decompressive lumbar laminectomy most commonly involving the L4–L5 level. On occasion, for those with foraminal/far lateral pathology at the level of the olisthesis, patients may require additional noninstrumented or instrumented lumbar fusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Epstein NE, Epstein JA, Carras R, Lavine LS. Degenerative spondylolisthesis with an intact neural arch: A review of 60 cases with an analysis of clinical findings and the development of surgical management. Neurosurgery. 1983. 13: 555-61
2. Epstein NE. Surgical management of lumbar stenosis: Decompression and indications for fusion. Neurosurg Focus. 1997. 3: e1-
3. Epstein ND. Commentary on: Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis by Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. NEJM 2016;374 (15):1424-34. Surg Neurol Int. 2016. 22: 7-
4. Epstein NE. Commentary on: A randomized controlled trial of fusion surgery for lumbar spinal stenosis (Forsth P, Ólafsson G, Carlsson T, Frost A, Borgström F, Fritzell P, et al. N Eng J Med 2016;374:1414-23). Surg Neurol Int. 2016. 7: S641-3
5. Försth P, Ólafsson G, Carlsson T, Frost A, Borgström F, Fritzell P. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med. 2016. 374: 1413-23
6. Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med. 2016. 374: 1424-34