- Professor of Clinical Neurosurgery, School of Medicine, University of State of New York at Stony Brook, New York, USA
- Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Professor of Clinical Neurosurgery, School of Medicine, University of State of New York at Stony Brook, New York, USA
Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_408_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Nursing review of spinal meningiomas. 16-Feb-2018;9:41
How to cite this URL: Nancy E. Epstein. Nursing review of spinal meningiomas. 16-Feb-2018;9:41. Available from: http://surgicalneurologyint.com/surgicalint-articles/nursing-review-of-spinal-meningiomas/
Abstract
Background:Spinal meningiomas are found in patients typically between the ages of 75 and 84: some report the average age to be 50. They occur with an incidence of approximately 1000 patients per year in the US, are mostly single (90%) rather than multiple (10%), and arise from the spinal meninges (arachnoid/dura). Tumors are typically posterior/posterolateral (70%) in location, leaving the remaining 30% in the anterior/anterolateral spinal canal. They produce symptoms and signs of radiculopathy (nerve root) and/or myelopathy (cord compression) depending on their site of origin.
Methods:Meningiomas may be single/sporadic (90%) or multifocal. They may arise primarily/spontaneously, can be radiation-induced, or associated with neurofibromatosis. They are found most frequently in females vs. males in up to a 3.4:1 ratio, occur predominantly in the thoracic spine. They are found in decreasing order in the cervical and lumbar spinal canals. The diagnosis of a meningioma is based on magnetic resonance (MR) studies, where tumors are isointense on T1 weighted MR, and hyperintense onf T2-weighted MR images; they also typically uniformly enhance with Gd-DTPA. On computed tomography (CT) examinations, they are usually characterized as calcified/hyperdense.
Results:The neurological deficits resulting from meningiomas and the rapidity of symptom/sign progression dictate whether they are treated surgically or nonsurgically. Management choices include; stereotactic radiation therapy only, and/or in combination with varied surgical resection techniques.
Conclusions:The majority of benign spinal cord tumors are meningiomas (40%) that are predominantly found in the thoracic spine in middle-aged females. Tumor levels (e.g. in descending order cervical, thoracic, lumbar), and their location (e.g. anterior/anterolateral 30%; dorsal/dorsolateral 70%) best determine whether nonoperative, operative, and/or operative intervention combined with routine vs. stereotactic radiosurgery are warranted.
Keywords: CT, Diagnostic studies, locations, MR, prognosis, spinal meningiomas, surgical techniques, tumors, stereotactic radiosurgery/Cyberknife, routine radiation
INTRODUCTION
There are approximately 1,000 spinal meningiomas treated per year in the USA.[
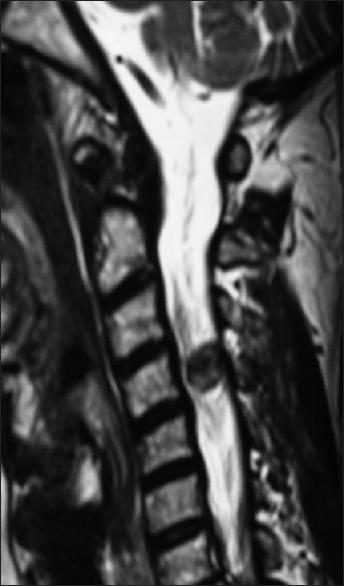
Figure 6
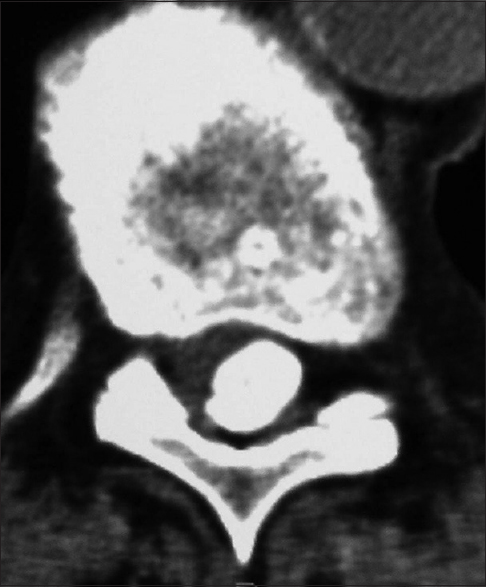
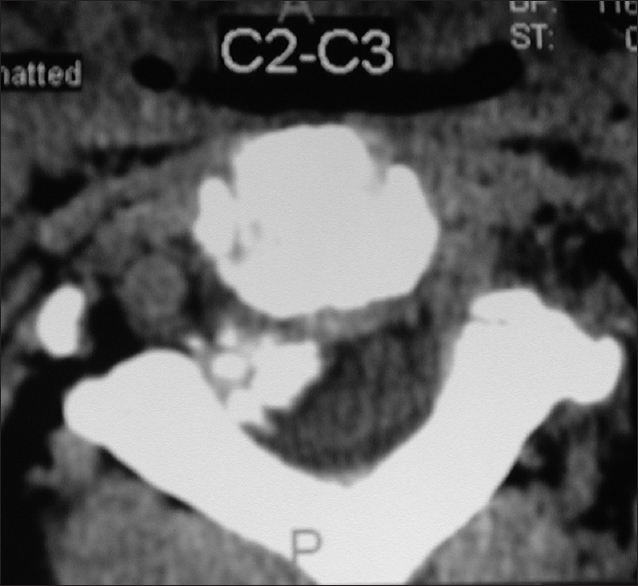
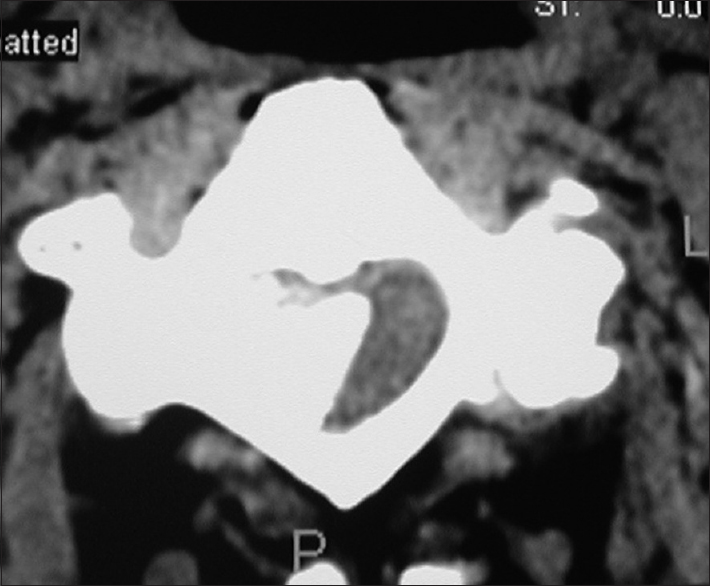
Axial soft tissue window CT scan at the C2-C3 level demonstrating inhomogeneous ossification/calcification of a meningioma (combined width some soft tissue/hypointense elements) filling the anterolateral and dorsolateral right side of the spinal canal. There is further central and right foraminal extension. Note the lesions appears to extend all the way from the lamina posteriorly to the disc space ventrally
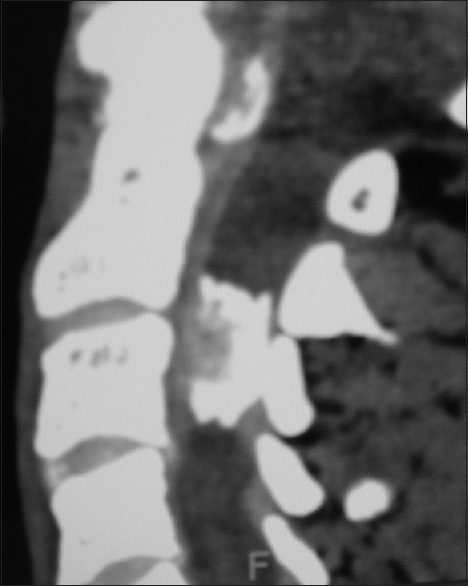
Figure 7
For the same patient depicted in
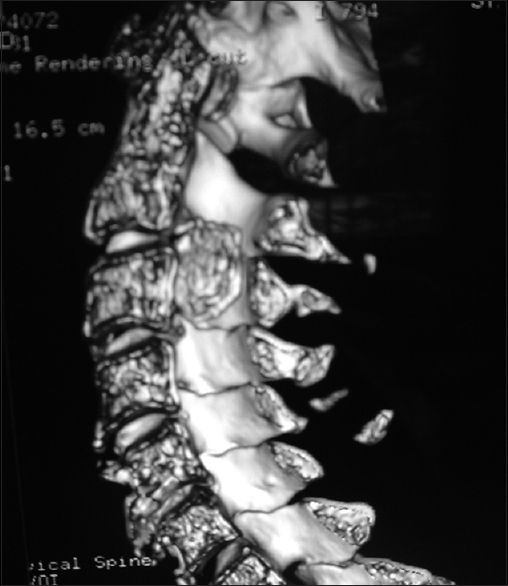
Figure 9
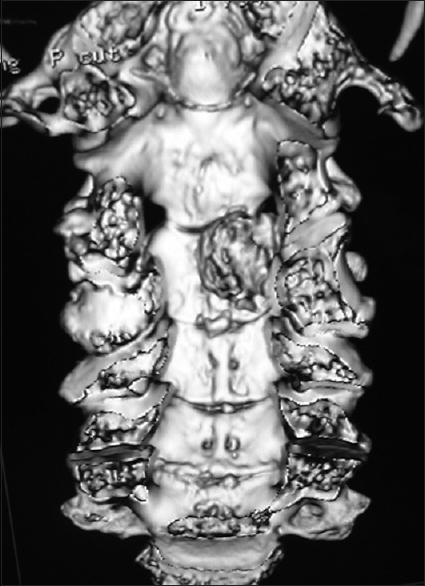
Coronal 3D CT taken from the same patient depicted in Figures
Location
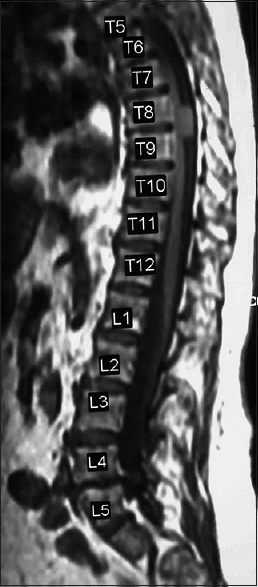
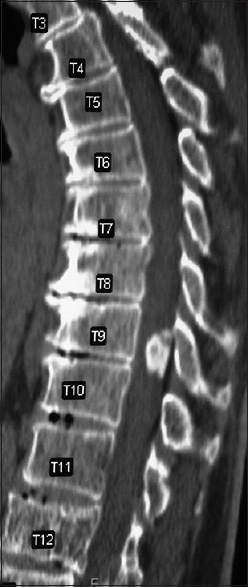
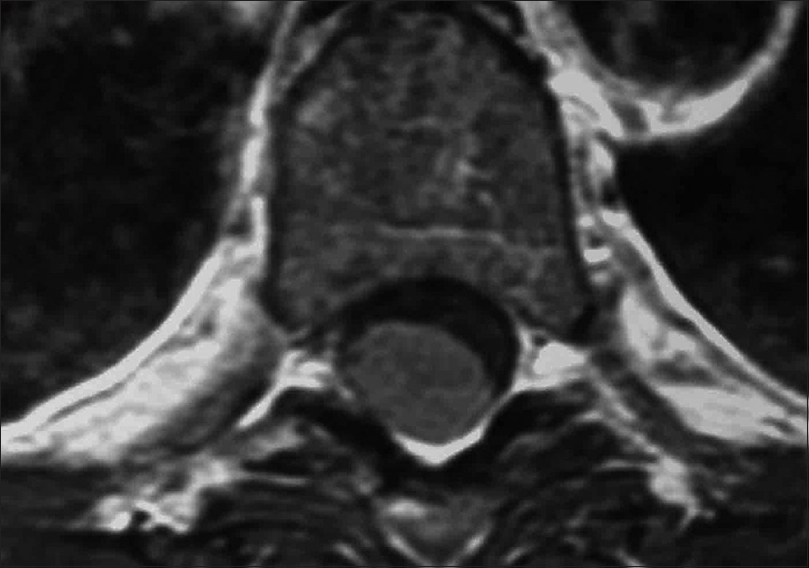
Most spinal meningiomas are intradural (inside the dura) and extramedullary (outside the spinal cord) [Figures
Epidemiology
Spinal meningiomas compromise 40% of all benign spinal tumors; the remainder consist predominantly of neurofibromas/schwannomas [Figures
FACTORS CONTRIBUTING TO MENINGIOMAS
Estrogen may contribute to the more aggressive presentation and higher recurrence rates for meningiomas in females. Their occurrence may also be attributed/linked to spinal trauma (e.g., involving the bony elements/meninges). Other etiologies include prior radiation exposure, or a history of neurofibromatosis (e.g., heterozygosity of the NF2 gene).
Diagnostic studies
Magnetic resonance examinations
MR studies are best at defining the location and extent of meningiomas. These lesions are isointense on T1-weighted, and hyperintesne on T2-weighted studies; they typically uniformly enhance with gadolinium DPTA. Classically, meningiomas demonstrate a clear dural base with the “dural tail” or “en-plaque” signs seen in up to 93% of cases. This finding does not, however, necessarily indicate dural penetrance.
Computed tomography studies
On CT examinations, meningiomas may demonstrate varying degrees of calcification. Sometimes they are uniformly calcified (57%), while at other times, there are only inhomogeneous areas of punctate ossification. CT may also signal whether there is dural penetration. Furthermore, MR and CT studies together may reveal foraminal extension (71%), adherence to the underlying nerve roots (79%), and/or intradural invasion (57%).
CLINICAL DEFICITS CORRELATING WITH MENINGIOMAS
The clinical symptoms/signs of meningioms correlate with their location in the spinal column. Their neurological deficits may reflect root compression (radiculopathy) and/or cord compression (myelopathy-diffuse weakness, hyperactive reflexes, loss of sensation including pin, position, and vibratory appreciation). Patients with spinal meningiomas may uniquely complain of increased pain at night that is relieved when sitting/standing typically during the day. Depending on their location, anterior/anterolateral lesions may contribute to weaknness without pain (myelopathy), whereas dorsolateral tumors (behind) may produce a loss of vibration/position sensation before exhibiting motor deficits.
UTILITY OF PREOPERATIVE SPINAL ANGIOGRAMS
As spinal meningiomas are typically very vascular lesions, a preoperative spinal angiogram with embolization of major vascular feeders may decrease the risk of intraoperative bleeding. Nevertheless, the risks of spinal angiography, that includes the potential for a stroke to the artery of Adamkiewicz (located T8-L3 if directly injected resulting in paraparesis/paraplegia), must be weighed against the benefits of less intraoperative hemorrhaging.
Surgery
Many types of spinal operations may be performed to resect spinal meningiomas. Lesions that are posterior/posterolateal (70%) are more readily resected utilizing a routine laminectomy. For the more anterior/anterolateral (30%) cervical/thoracic tumors, a costotransversectomy (removal of a part of the rib), extracavitary (very lateral) approach, and/or anterior approaches may be warranated to achieve tumor resection without cord manipulation. Depending upon the extent and location of tumor removal (e.g. cervicothoracic junction and thoracolumbar junction), an additional fusion may be required.
DURAL CLOSURE TECHNIQUES FOR RESECTION OF INTRADURAL LESIONS
Advantages of dorsal/dorsolateral approaches to cervical, thoracic, or lumbar meningiomas include the avoidance of a persistent cerebrospinal fluid leak. Here, in most cases, the dura, following tumor removal, may be clearly elevated/everted, allowing for clear placement of 7-0 Gortex sutures (note the needle is smaller than the suture that is nonresorbable). If the edges cannot be brought together without tension or if the tumor extends to and through the dura requiring dural resection, then a dural patch graft may be needed. One may use bovine pericardium or a fascial autograft to close the dura in some of these latter cases. Closure is then enhanced with microdural staples, a fibrin sealant, and microfibrillar collagen.
Dural penetrance vs. dural-sparing meningiomas
Some meningiomas will extend to/through the dura whereas others will involve the inner dural membrane while sparing the external sheath. If possible, preserving the dura and obtaining a primary closure is preferable to placing a dural patch graft as the latter carries a higher risk of persistent CSF fistula.
Pathology
There are several pathologic types of meningiomas; 70% psammomatous, 15% meningothelial, 7% angiomatous, 7% transitional, and 1% may be cancerous/infiltrative/malignant (e.g., meningiopericytomas, clear cell meningiomas) [Figures
RADIATION THERAPY AND RADIOSURGERY
Increasingly, stereotactic radiosurgery may be added to the resection of spinal meningiomas or may indeed supplant the need for surgery. This may be particularly true for the most complex craniocervical lesions. Although surgical resection is often considered optimal by most surgeons, the size/location of the lesion, pattern of growth, and age/comorbidities of the patient may dictate the utilization of nonoperative/steretotactic radiosurgical treatment. In addition, radiosurgery may be the treatment of choice for recurrent/residual tumors (e.g., difficult to resect). At present, sophisticated stereotactic radiosurgery techniques may be used as the primary radiosurgery treatment, or in other cases, to supplement where prior conventional fractionated radiotherapy was delivered.
Radiation-induced myelopathy
Patients who have undergone radiation therapy for spinal meningiomas may develop the late onset (6 months to 2 years later) or radiation-induced myelopathy (0.2–5%). This is typically dose dependent, and may be best diagnosed on T2-weighted MR studies. Treatment options include steroids and/or nerve transmitter medications.
CONCLUSION
Spinal meningiomas are most frequently found in the thoracic, followed by the cervical and lumbar spine, and constitute approximately 40% of all benign spinal tumors [Figures
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bayoumi AB, Laviv Y, Yokus B, Efe IE, Toktas ZO, Kilic T. Proposal of a new radiological classification system for spinal meningiomas as a descriptive tool and surgical guide. Clin Neurol Neurosurg. 2017. 162: 118-26
2. Bocchetti A, Cioffi V, Gragnaniello C, de Falco R, Levy WJ, Bay J. Versatility of sub-occipital approach for foramen magnum meningiomas: A single centre experience. J Spine Surg. 2017. 3: 411-8
3. Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Benzel EC, Barnholtz-Sloan JS. Descriptive Epidemiology of Spinal Meningiomas in the United States. Spine (Phila Pa 1976). 2015. 40: E886-9
4. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010. 99: 379-91
5. Roux FX, Nataf F, Pinaudeau M, Borne G, Devaux B, Meder JF. Intraspinal meningiomas: Review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996. 46: 458-63
6. Zhang LH, Yuan HS. Imaging Appearances and Pathologic Characteristics of Spinal Epidural Meningioma. AJNR Am J Neuroradiol. 2017. p.