- Department of Neuroscience, Winthrop University Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Department of Neuroscience, Winthrop University Hospital, Mineola, New York, USA
DOI:10.4103/2152-7806.174892
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Epstein NE. Older literature review of increased risk of adjacent segment degeneration with instrumented lumbar fusions. Surg Neurol Int 25-Jan-2016;7:
How to cite this URL: Epstein NE. Older literature review of increased risk of adjacent segment degeneration with instrumented lumbar fusions. Surg Neurol Int 25-Jan-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/older-literature-review-of-increased-risk-of-adjacent-segment-degeneration-with-instrumented-lumbar-fusions/
Abstract
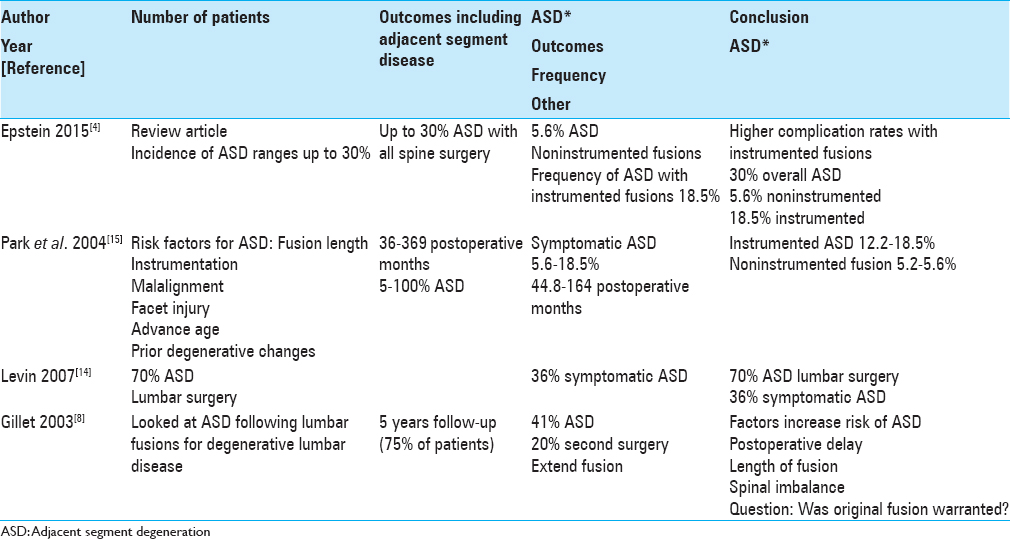
Background:Adjacent segment degeneration (ASD) following lumbar spine surgery occurs in up to 30% of cases, and descriptions of such changes are not new. Here, we review some of the older literature concerning the rate of ASD, typically more severe cephalad than caudad, and highly correlated with instrumented fusions. Therefore, for degenerative lumbar disease without frank instability, ASD would be markedly reduced by avoiding instrumented fusions.
Methods:In a prior review, the newer literature regarding the frequency of ASD following lumbar instrumented fusions (e.g., transforaminal or posterior lumbar interbody fusions [TLIF/PLIF] fusions or occasionally, posterolateral fusions [PLFs]) was presented. Some studies cited an up to an 18.5% incidence of ASD following instrumented versus noninstrumented fusions/decompressions alone (5.6%). A review of the older literature similarly documents a higher rate of ASD following instrumented fusions performed for degenerative lumbar disease alone.
Results:More frequent and more severe ASD follows instrumented lumbar fusions performed for degenerative lumbar disease without instability. Alternatively, this entity should be treated with decompressions alone or with noninstrumented fusions, without the addition of instrumentation.
Conclusions:Too many studies assume that TLIF, PLIF, and even PLF instrumented fusions are the “gold standard of care” for dealing with degenerative disease of the lumbar spine without documented instability. It is time to correct that assumption, and reassess the older literature along with the new to confirm that decompression alone and noninstrumented fusion avoid significant morbidity and even potentially mortality attributed to unnecessary instrumentation.
Keywords: Adjacent level disease, lumbar fusions, unnecessary
INTRODUCTION
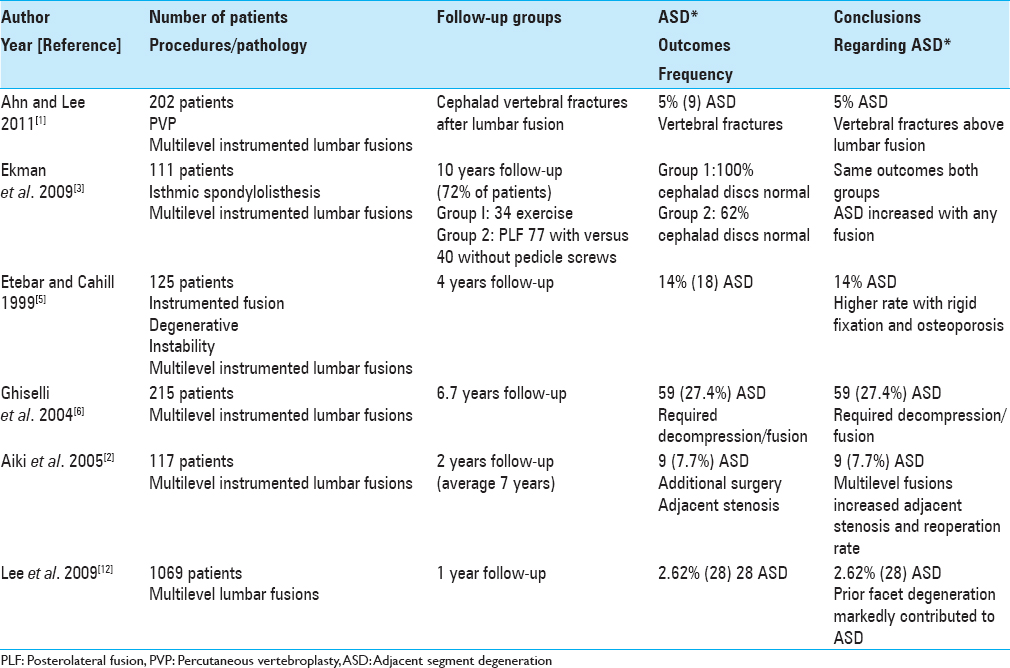
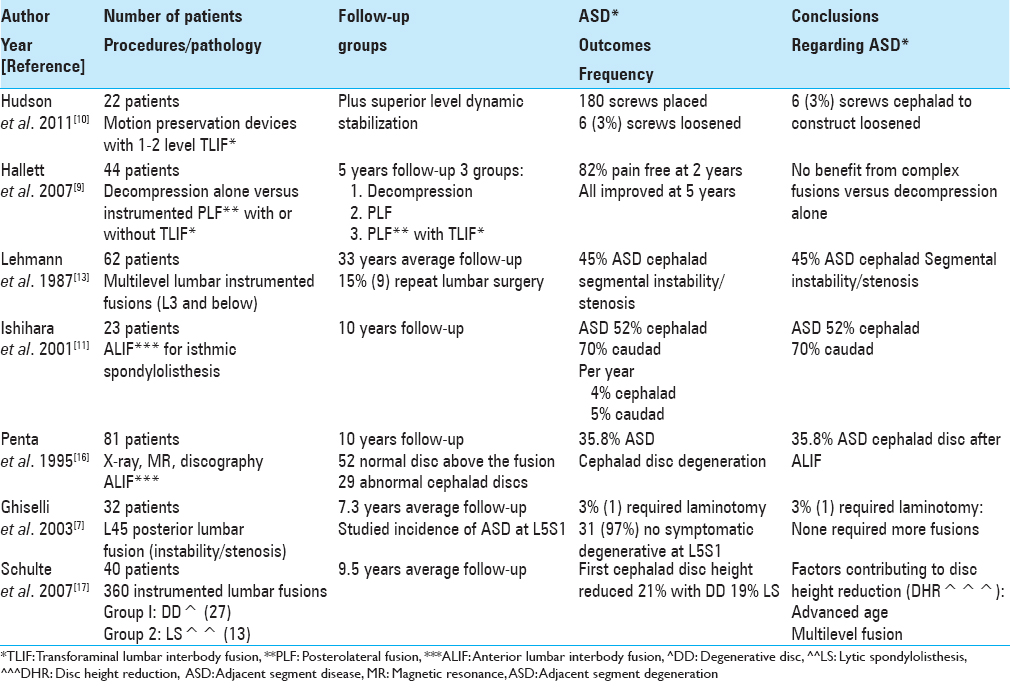
Adjacent segment degeneration (ASD) is defined as “the mobile segment next to a lumbar or lumbosacral spine fusion” [Tables
FREQUENCY OF ASD AFTER LUMBAR FUSION
Varying frequencies of ASD have been reported following lumbar fusions, typically utilizing instrumentation (e.g.,14–70%) [Tables
Comments
The frequency of ASD following instrumented lumbar fusions varies markedly from 14% to 70% as acknowledged above [Tables
THE FATE OF THE ADJACENT MOTION SEGMENTS AFTER LUMBAR FUSION
In 2003, Gillet followed outcomes of lumbar fusions addressing degenerative lumbar disease over a 2–15 postoperative interval; 75% of patients had a minimum 5-year follow-up [
Comments
This 5 year study following instrumented lumbar fusions performed for degenerative lumbar disease, noted a 41% incidence of ASD and 20% frequency of secondary surgery requiring extension of fusions.[
CLINICAL OUTCOME ANALYSIS OF ASD AT L5S1 FOLLOWING AN L4–L5 ISOLATED FUSION
In 2003, Ghiselli et al. retrospectively evaluated the caudad ASD motion at L5–S1 following focal L4–5 posterior lumbar fusions (average follow-up 7.3 years; range 2.3–12.4 years) [
Comments
For the initial small group of just 32 patients in 2003, the incidence of ASD at the L5–S1 level below an L4–L5 fusion was just 3%.[
HIGH FREQUENCY/SEVERITY OF ASD FOLLOWING INSTRUMENTED LUMBAR FUSIONS FOR DEGENERATIVE LUMBAR DISEASE
In 2004, Park et al. reviewed, over 44.8–164 postoperative months, the frequency/indications and risk factors resulting in adjacent segment degeneration in the lumbar spine following decompressions alone or noninstrumented (5.2–5.6%) fusions versus pedicle screw instrumented fusions (12.2–18.5%) for patients with degenerative spine disease without instability [
Comments
In 2004, Park et al. determined the frequency of ASD following decompressions alone/noninstrumented (5.2–5.6%) fusions was much less than for pedicle screw instrumented fusions (12.2–18.5%) in patients with degenerative spine disease without instability.[
SYMPTOMATIC ADJACENT SEGMENT STENOSIS FOLLOWING LUMBAR FUSIONS REQUIRING SECONDARY SURGERY
In 2005, Aiki et al. observed that although ASD is frequently noted following lumbar fusions, few patients are typically sufficiently symptomatic to warrant secondary surgery [
Comments
In 2005, Aiki et al. observed that 9 (7.7%) of 117 patients initially undergoing lumbar fusions developed symptomatic adjacent level stenosis requiring secondary surgery.[
DISC HEIGHT REDUCTION AT ADJACENT SEGMENTS AND CLINICAL OUTCOME 10 YEARS AFTER LUMBAR 360 FUSIONS
In 2007, Schulte et al. looked at the frequency of ASD following 40 lumbar fusions (360 instrumented procedures); there were 27 patients in Group 1 (degenerative disc disease), and 13 in Group 2 (lytic spondylolisthesis) who were evaluated over 114 postoperative months [
Comments
In 2007, Schulte et al. looked at the frequency of ASD following 40 lumbar fusions (360 instrumented procedures performed for degenerative disc disease 27 patients) versus lytic spondylolisthesis (13 patients) over 114 postoperative months.[
ADJACENT SEGMENT DEGENERATION FOLLOWING SPINAL FUSION FOR DEGENERATIVE DISC DISEASE
In 2007, Levin et al. noted the incidence of ASD in the cervical spine after a fusion approaches 50%, while in the lumbar spine it is 70% at 10 postoperative years [
Comments
In 2007, Levin et al. noted the frequency of radiographic ASD 10 years following both cervical (50%) and lumbar (70%) surgery, and correctly emphasized that symptomatic disease occurs in fewer patients, respectively, 25% and 36%.[
FORAMINAL STENOSIS AND SINGLE-LEVEL DISC DISEASE: DECOMPRESSION VERSUS DECOMPRESSION/INSTRUMENTED FUSION
In 2007, Hallett et al. studied the 5 year outcomes for 44 patients randomly divided into three surgical groups; spinal decompressions alone (Group 1), decompression and instrumented posterolateral fusion (PLF) (with pedicle screw fixation) (Group 2), and instrumented PLF (pedicle/screw fixation) plus transforaminal lumbar interbody fusion (TLIF) using titanium cages filled with autograft (Group 3) [
Comments
In 2007, Hallett et al. studied the 5 year outcomes for 44 patients randomly divided into those undergoing decompressions alone, decompression and instrumented PLF (with pedicle screws), and instrumented PLF with pedicle screws plus TLIF using titanium cages filled with autograft (Group 3).[
LONG-TERM EFFECT OF LUMBAR FUSION ON ADJACENT DISC DEGENERATION
In 2009, Ekman et al. noted that randomized controlled trials (RCTs) previously documented that ASD is accelerated following lumbar fusions [
Comments
In 2009, Ekman et al. noted that RCTs previously documented that ASD is accelerated following lumbar fusions [
RISK FACTORS FOR ASD AFTER LUMBAR FUSION
In 2009, Lee et al. utilizing MR studies, looked at the frequency and clinical risk factors contributing to ASD 1 year following lumbar/lumbosacral fusions performed for degenerative disease (1995–2006) [
Comments
In 2009, Lee et al. found a 2.62% risk of ASD in 1069 patients following lumbar/lumbosacral fusions performed for degenerative disease.[
VERTEBROPLASTY TO TREAT ADJACENT VERTEBRAL FRACTURES AFTER LUMBAR INTERBODY FUSION
In 2011, Ahn and Lee looked at the incidence of cephalad adjacent segment osteoporotic vertebral compression fractures following lumbar interbody fusions, and offered percutaneous vertebroplasty (PVP) to address these iatrogenic injuries [
Comments
Ahn and Lee concluded that ASD resulting in vertebral osteoporotic fractures occurring cephalad to instrumented fusions could be effectively managed with PVP.[
HYBRID LUMBAR DYNAMIC STABILIZATION WITH POSTERIOR SPINAL FUSION
In 2011, Hudson et al. mistakenly stated that instrumented lumbar fusions are the “gold standard” for treating lumbar degenerative disc disease; indeed this is an ill-founded premise [
Comments
In 2011, Hudson et al. mistakenly stated that instrumented lumbar fusions are the “gold standard” for treating lumbar degenerative disc disease; indeed this is an ill-founded premise [
SUMMARY
The frequency of ASD following instrumented spinal fusions (up to 18.5%) far exceeds that of ASD when decompressions alone and/or noninstrumented fusions (up to 5.6%) are performed.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn Y, Lee SH. Vertebroplasty for adjacent vertebral fracture following lumbar interbody fusion. Br J Neurosurg. 2011. 25: 104-8
2. Aiki H, Ohwada O, Kobayashi H, Hayakawa M, Kawaguchi S, Takebayashi T. Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci. 2005. 10: 490-5
3. Ekman P, Möller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009. 18: 1175-86
4. Epstein NE. Adjacent level disease following lumbar spine surgery. A review. Surg Neurol Int Spine. 2015. p.
5. Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999. 90: 163-9
6. Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004. 86-A: 1497-503
7. Ghiselli G, Wang JC, Hsu WK, Dawson EG. L5-S1 segment survivorship and clinical outcome analysis after L4-L5 isolated fusion. Spine (Phila Pa 1976). 2003. 28: 1275-80
8. Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003. 16: 338-45
9. Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: A randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976). 2007. 32: 1375-80
10. Hudson WR, Gee JE, Billys JB, Castellvi AE. Hybrid dynamic stabilization with posterior spinal fusion in the lumbar spine. SAS J. 2011. 5: 36-43
11. Ishihara H, Osada R, Kanamori M, Kawaguchi Y, Ohmori K, Kimura T. Minimum 10-year follow-up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. J Spinal Disord. 2001. 14: 91-9
12. Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009. 18: 1637-43
13. Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY. Long-term follow-up of lower lumbar fusion patients. Spine (Phila Pa 1976). 1987. 12: 97-104
14. Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007. 65: 29-36
15. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976). 2004. 29: 1938-44
16. Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995. 20: 743-7
17. Schulte TL, Leistra F, Bullmann V, Osada N, Vieth V, Marquardt B. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007. 16: 2152-8