- Department of Neurosciences, Division of Neurological Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
Correspondence Address:
Ayman Bahatheq
Department of Neurosciences, Division of Neurological Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
DOI:10.4103/2152-7806.187495
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abou-Al-Shaar H, Bahatheq A, Takroni R, Al-Thubaiti I. Optic chiasmal cavernous angioma: A rare suprasellar vascular malformation. Surg Neurol Int 01-Aug-2016;7:
How to cite this URL: Abou-Al-Shaar H, Bahatheq A, Takroni R, Al-Thubaiti I. Optic chiasmal cavernous angioma: A rare suprasellar vascular malformation. Surg Neurol Int 01-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/optic-chiasmal-cavernous-angioma-rare-suprasellar-vascular-malformation/
Abstract
Background:Suprasellar cavernous malformation in the optic pathway is not commonly encountered. To date, there are only few reports present in the literature.
Case Description:The authors report a rare case of suprasellar optic pathway cavernous malformation in a 33-year-old female who presented with progressive visual loss. Her imaging revealed a large heterogeneous, hyperintense, hemorrhagic right suprasellar extra-axial complex cystic structure, causing mass effect on the adjacent hypothalamus and third ventricle displacing these structures. Gross total resection of the lesion was achieved utilizing a right frontal craniotomy approach. Histopathological examination confirmed the diagnosis of suprasellar chiasmal cavernous malformation.
Conclusion:Although visual pathway cavernous malformation is a rare event, it should be included in the differential diagnosis of lesions occurring suprasellarly in the visual pathway and hypothalamus.
Keywords: Cavernoma, cavernous angioma, hypothalamus, optic pathway, suprasellar region
INTRODUCTION
Cavernous malformations (CMs) are common low-flow lesions of the central nervous system (CNS), accounting for 10–20% of all vascular malformations with an incidence of 0.3–0.7% in the general population.[
CASE REPORT
History and examination
A 33-year-old female presented 3 months postpartum with a headache of moderate severity and progressive visual loss in both eyes. On examination, the patient's Glasgow coma scale (GCS) was 15/15. Visual field examination showed left homonymous incomplete hemianopia. Her visual acuity was 20/25 in the right eye and 20/30 in the left eye. Her discs and macula were healthy bilaterally. Extraocular movements were intact and pupils were reactive. The rest of her examination was unremarkable. Complete endocrine workup was normal.
Imaging
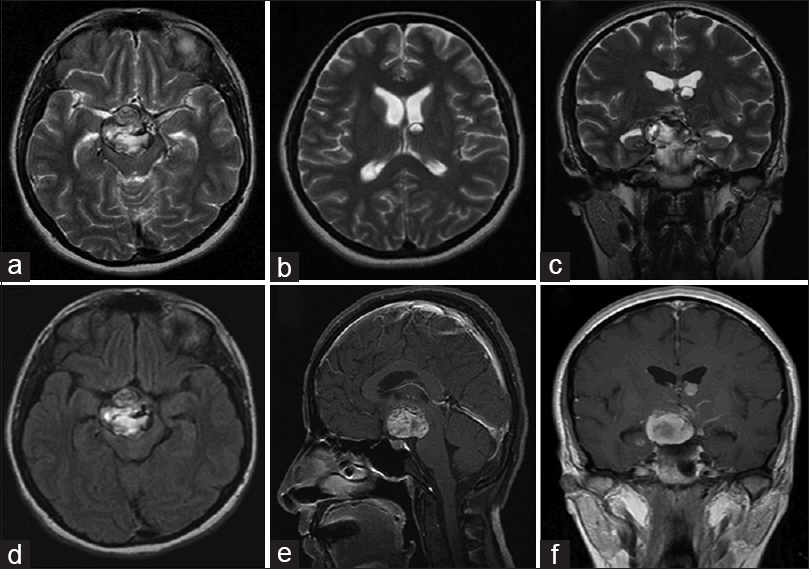
Magnetic resonance imaging (MRI) revealed a large heterogeneous, hyperintense, hemorrhagic right suprasellar extra-axial complex cystic structure measuring 31 × 30 × 90 mm on T1-weighted images. There was mass effect on the adjacent hypothalamus and third ventricle displacing them toward the left and superiorly in addition to the optic pathway. The pituitary stalk was displaced toward the left. The lesion encased the right posterior cerebral artery and displaced the right carotid artery laterally [
Figure 1
Preoperative magnetic resonance imaging of the brain demonstrating a heterogeneous hyperintense T1 and T2 suprasellar mass compatible with acute/subacute hemorrhagic lesion measuring 3.1 × 2.9 × 2.1 cm with no clear evidence of enhancement allowing for intrinsic T1 hyperintensity (a, c, d). There is some adjacent extension of blood products into the right hippocampal sulcus (c). There is also a left-sided moderately-sized venous angioma in the basal ganglia (c, f). This represents a large hypothalamic region/subependymal cavernous angioma with recent hemorrhage and associated surrounding mild edema mainly in the right basal ganglia and thalamus. Moreover, displacement and mass effect on the surrounding structures including basal ganglia, subcapsular brain parenchyma, as well as hypothalamus and displacement of the cerebral peduncle with a mass effect on the midbrain is noted (a, c, d-f). An additional hemorrhagic focus in the left frontal horn of the lateral ventricle close to the foramen of Monro region is also seen (b, c). Specifically, there is a mass effect and anterior displacement of the partially visualized pituitary infundibulum as well as a mild mass effect on the optic chiasm particularly on the right side compressing and displacing it (c, e, f). No additional susceptibility foci are noted
Operation and histopathological findings
The patient underwent right frontal craniotomy and gross total resection of her suprasellar intrachiasmatic large infiltrative hemorrhagic CM. Organizing blood clots with reactive fibrohistiocytic and inflammatory reaction admixed with some ectatic vascular channels suggestive of a vascular malformation were noted. There were small foci admixed with granulation tissue, showing some dilated cavernous spaces that would be compatible with a vascular malformation such as cavernous angioma. On immunohistochemistry, the lesion was CD163+, CD20 rare, CD3+, CD34+, CD31+, CD38+, CTK−, EMA plasma cells, GFAP−, S100 dendritic cells, SMA vascular smooth muscle.
Postoperative course
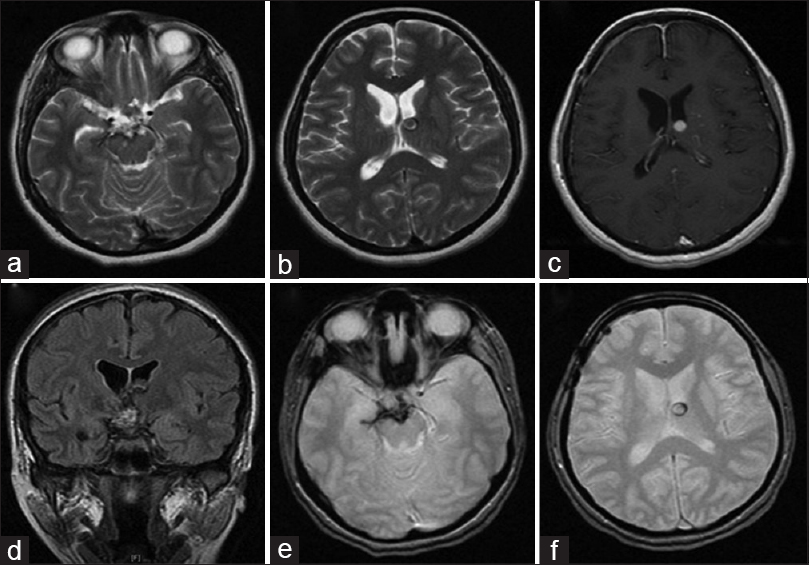
The patient had an uneventful operative course. Her visual acuity improved to 20/20 in both eyes. Extraocular muscles showed mild limitation of both eyes in an upward gaze. Otherwise, she was stable with no neurological deficits. Follow-up MRI at 12 months revealed complete removal of the suprasellar hemorrhagic CM with no evidence of a residual lesion or recurrence [
Figure 2
Postoperative magnetic resonance imaging of the brain depicting complete removal of the suprasellar hemorrhagic cavernous malformation with no evidence of a residual lesion or recurrence with some adjacent extension of blood products into right hippocampal sulcus (a-d) and no hemosiderin staining of the ventricular surface on the gradient-echo imaging (e, f)
DISCUSSION
Optic pathway CMs are rare benign vascular lesions, representing less than 1% of all CNS CMs.[
The pathogenesis of CMs remains elusive. They are thought to arise during the early periods of embryogenesis and grow according to blood changes and malformative mechanisms.[
The natural history of optic pathway CMs is also not clear. It is thought that optic pathway CMs have a higher tendency to bleed than cerebral ones because of the eloquence of the optic pathway region.[
It is not easy to diagnose CMs of the optic pathway preoperatively. It is not uncommon for them to be misdiagnosed as optic neuritis, and a great number of these patients receive corticosteroid therapy.[
MRI is considered the most sensitive and specific imaging modality for the diagnosis of CM.[
It is important to note that none of these imaging features is pathognomonic as they can be encountered in other conditions. Thus, preoperative diagnosis of optic pathway CMs is extremely difficult and challenging. CMs of the optic pathway are commonly misdiagnosed as optic neuritis, optic glioma, meningioma, craniopharyngioma, venous angioma, arteriovenous malformation, thrombosed aneurysm, and pituitary apoplexy.[
The treatment of choice for optic pathway CM is complete surgical resection of the lesion. Surgical resection of these lesions is considered a challenge because of their deep location and eloquence. Complete surgical resection of the CM is essential in order to prevent regrowth and bleeding.[
The surgical approach should allow optimal exposure of the lesion using the shortest route and with minimal brain retraction. Various surgical approaches have been reported in the literature including pterional, orbitozygomatic, supraorbital, subfrontal, and transbasal interhemispheric approaches. Almost half of the cases reported in the literature were managed through the frontotemporal approach.[
Spontaneous recovery has been reported in the literature, especially in children.[
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflicts of interest regarding the production of this article. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
1. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995. 83: 56-9
2. Buonaguidi R, Canapicci R, Mimassi N, Ferdeghini M. Intrasellar cavernous hemangioma. Neurosurgery. 1984. 14: 732-4
3. Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg. 2007. 106: 379-83
4. Campbell PG, Jabbour P, Yadla S, Awad IA. Emerging clinical imaging techniques for cerebral cavernous malformations: A systematic review. Neurosurg Focus. 2010. 29: E6-
5. Cerase A, Franceschini R, Battistini S, Maria Vallone I, Penco S, Venturi C. Cavernous malformation of the optic nerve mimicking optic neuritis. J Neuroophthalmol. 2010. 30: 126-31
6. de Champfleur NM, Langlois C, Ankenbrandt WJ, Le Bars E, Leroy MA, Duffau H. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011. 68: 641-7
7. Deshmukh VR, Albuquerque FC, Zabramski JM, Spetzler RF. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. 2003. 53: 352-7
8. Hayashi S, Kondoh T, Morishita A, Sasayama T, Sanabria EA, Kohmura E. Congenital cavernous angioma exhibits a progressive decrease in size after birth. Childs Nerv Syst. 2004. 20: 199-203
9. Hempelmann RG, Mater E, Schröder F, Schön R. Complete resection of a cavernous haemangioma of the optic nerve, the chiasm, and the optic tract. Acta Neurochir. 2007. 149: 699-703
10. Jo KW, Kim SD, Chung EY, Park IS. Optochiasmatic cavernous angioma with rapid progression after biopsy despite radiation therapy. J Korean Neurosurg Soc. 2011. 49: 120-3
11. Lehner M, Fellner FA, Wurm G. Cavernous haemangiomas of the anterior visual pathways. Short review on occasion of an exceptional case. Acta Neurochir. 2006. 148: 571-88
12. Little JR, Awad IA, Jones SC, Ebrahim ZY. Vascular pressures and cortical blood flow in cavernous angioma of the brain. J Neurosurg. 1990. 73: 555-9
13. Liu JK, Lu Y, Raslan AM, Gultekin SH, Delashaw JB. Cavernous malformations of the optic pathway and hypothalamus: Analysis of 65 cases in the literature. Neurosurg Focus. 2010. 29: E17-
14. Maitland CG, Abiko S, Hoyt WF, Wilson CB, Okamura T. Chiasmal apoplexy. Report of four cases. J Neurosurg. 1982. 56: 118-22
15. Martínez-Lage JF, de la Fuente I, Ros de San Pedro J, Fuster JL, Pérez-Espejo MA, Herrero MT. Cavernomas in children with brain tumors: A late complication of radiotherapy. Neurocirugia. 2008. 19: 50-4
16. Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K. The natural history of cavernous malformations: A prospective study of 68 patients. Neurosurgery. 1999. 44: 1166-1171
17. Muta D, Nishi T, Koga K, Yamashiro S, Fujioka S, Kuratsu J. Cavernous malformation of the optic chiasm: Case report. Br J Neurosurg. 2006. 20: 312-5
18. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006. 21: e4-
19. Notelet L, Houtteville JP, Khoury S, Lechevalier B, Chapon F. Proliferating cell nuclear antigen (PCNA) in cerebral cavernomas: An immunocytochemical study of 42 cases. Surg Neurol. 1997. 47: 364-70
20. Ozer E, Kalemci O, Yücesoy K, Canda S. Optochiasmatic cavernous angioma: Unexpected diagnosis. Case report. Neurol Med Chir. 2007. 47: 128-31
21. Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT. Cavernous malformations of the brainstem: Experience with 100 patients. J Neurosurg. 1999. 90: 50-8
22. Rheinboldt M, Blase J. Exophytic hypothalamic cavernous malformation mimicking an extra-axial suprasellar mass. Emerg Radiol. 2011. 18: 363-367
23. Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987. 67: 518-24
24. Rigamonti D, Johnson PC, Spetzler RF, Hadley MN, Drayer BP. Cavernous malformations and capillary telangiectasia: A spectrum within a single pathological entity. Neurosurgery. 1991. 28: 60-4
25. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991. 75: 709-14
26. Shibuya M, Baskaya MK, Saito K, Suzuki Y, Ooka K, Hara M. Cavernous malformations of the optic chiasma. Acta Neurochir. 1995. 136: 29-36
27. Shkarubo AN, Serova NK, Tropinskaia OF, Shishkina LV, Pronin IN. Chiasmatic cavernoma. Zh Vopr Neirokhir Im N N Burdenko. 2005. 2: 20-21
28. Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001. 94: 972-7
29. Tan T, Tee JW, Trost N, McKelvie P, Wang YY. Anterior visual pathway cavernous malformations. J Clin Neurosci. 2015. 22: 258-67
30. Tien R, Dillon WP. MR imaging of cavernous hemangioma of the optic chiasm. J Comput Assist Tomogr. 1989. 13: 1087-8