- Department of Neurosurgery, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Correspondence Address:
Nicholas Calvin, Department of Neurosurgery, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
DOI:10.25259/SNI_588_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nicholas Calvin, Renindra Ananda Aman. Optimizing glioblastoma treatment: A systematic review and meta-analysis of local injection and systemic drug delivery system in murine models. 22-Nov-2024;15:428
How to cite this URL: Nicholas Calvin, Renindra Ananda Aman. Optimizing glioblastoma treatment: A systematic review and meta-analysis of local injection and systemic drug delivery system in murine models. 22-Nov-2024;15:428. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13245
Abstract
Background: Glioblastoma (GBM) is an aggressive primary brain tumor with a poor prognosis. The current gold standard for GBM treatment, known as the Stupp protocol, includes maximal safe surgical resection followed by radiotherapy and temozolomide chemotherapy. Despite extending survival modestly, this regimen is associated with significant side effects and limited efficacy, resulting in a median survival (MS) of 15 months and a 5-year survival rate of only 7%. A major challenge in GBM treatment is the blood–brain barrier (BBB), which restricts the penetration of therapeutic agents into the brain, thereby limiting the effectiveness of systemic therapies. To address these limitations, this systematic review and meta-analysis investigated the effectiveness of injectable local drug delivery systems (DDS) compared to systemic DDS in murine GBM models. This study aimed to provide robust evidence supporting the potential benefits of injectable local DDS for GBM treatment.
Methods: A comprehensive literature search was conducted using PubMed, Embase, and ScienceDirect databases. The studies included were original research on local DDS of anticancer agents compared to systemic DDS in orthotopic GBM tumor models. The data extraction process included information on survival rates, tumor growth, and other relevant outcomes. Statistical analysis was performed using Review Manager 5.4, employing a random-effects model to calculate the pooled mean difference (MD) in survival time between local and systemic DDS.
Results: Out of 1341 records, six studies met the inclusion criteria, totaling 129 murine models. The meta-analysis revealed that local injection of DDS significantly improved the MS compared to systemic administration (MD = 2.76; 95% confidence interval, 0.43–5.09; P = 0.03; I2 = 93%). The local injection of the DDS bypassed the BBB, achieving higher local drug concentrations and sustained release at the tumor site, leading to enhanced therapeutic efficacy and reduced systemic toxicity.
Conclusion: This systematic review and meta-analysis provide compelling evidence that local injection of DDS significantly improves survival in GBM models compared with systemic therapies. These findings highlight the potential of local DDS to overcome the challenges posed by the BBB and deliver higher concentrations of therapeutic agents directly to the tumor site. However, further research is needed to validate these findings in human clinical trials and refine DDS formulations. Future research should focus on developing DDS formulations capable of delivering multiple therapeutic agents simultaneously, addressing the experimental variability in preclinical models, and conducting rigorous clinical trials to evaluate the safety and efficacy of local DDS in human patients. Standardizing the testing methods across studies will facilitate more accurate comparisons and data integration, ultimately advancing the clinical translation of this promising therapeutic approach.
Keywords: Blood–brain barrier, Drug delivery system, Glioblastoma, Survival, Mice
INTRODUCTION
Glioblastoma (GBM) is an aggressive and malignant primary brain tumor with a poor prognosis.[
One of the major challenges in treating GBM is the blood–brain barrier (BBB), a protective shield that restricts the penetration of therapeutic agents into the brain.[
To address these limitations, local drug-delivery systems (DDSs) have emerged as promising alternatives. Local DDS aims to deliver therapeutic agents directly to the tumor site, bypassing the BBB and achieving higher local drug concentrations.[
The development of injectable local DDS offers a potential solution to these limitations. Injectable DDS allows for precise and targeted delivery of therapeutic agents directly into the tumor, reducing off-target effects and maximizing drug concentration at the tumor site. This systematic review and meta-analysis aimed to assess the efficacy of injectable local DDS compared to systemic DDS in murine models of GBM. By comparing survival rates and other relevant outcomes, this study sought to provide robust evidence supporting the potential benefits of injectable local DDS in GBM treatment.[
In summary, while the Stupp protocol remains the standard of care for GBM, its limitations highlight the need for innovative treatment strategies.[
MATERIALS AND METHODS
Literature search
The systematic review conducted in this study employed a rigorous methodology to synthesize and analyze the existing literature on local DDS. The process began with a comprehensive search of relevant medical databases, including PubMed, Embase, and ScienceDirect, without any time limitations. We used specific search terms and MeSH descriptors such as “GBM,” “local drug delivery system,” “systemic drug delivery system,” “survival,” and “pre-clinical” to ensure a targeted and exhaustive retrieval of relevant studies. To enhance the thoroughness of our search, we meticulously examined the reference lists of the included studies to identify additional relevant research. Two authors independently screened abstracts and assessed full-text articles for eligibility, ensuring an unbiased selection and evaluation process. This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, guaranteeing a transparent and standardized approach.[
Eligibility
Following a systematic screening process, we evaluated the eligibility of articles in the initial pool. To be included, studies need to meet specific criteria, such as being written in English, containing original research on local DDS of anticancer agents compared to systemic anticancer DDS in orthotopic GBM tumor models, and involving interventions with injectable local DDS. To ensure a high level of evidence, articles focusing solely on in vitro and clinical studies without in vivo components were excluded from the study. Furthermore, review articles, case reports, case series, conference abstracts, and studies without available full text were excluded from the study.
Study selection
Two independent reviewers thoroughly assessed the titles and abstracts of each article according to predefined inclusion and exclusion criteria. After this initial evaluation, full texts of potentially eligible studies were obtained and reassessed to confirm that they met the inclusion criteria. Discussions were conducted to reach a consensus in cases of disagreement between the reviewers, thereby ensuring the integrity and impartiality of the selection process.
Data extraction
The selected studies underwent data extraction, which involved the collection of information on the author, year, country, cancer cell line, mouse model, number of implanted cells, anticancer drugs used, drug, delivery vehicle, mean survival of the local DDS, mean survival of the systemic DDS, and percentage of mean survival change. The data were systematically organized into a comprehensive database for further analysis. Subsequently, qualitative synthesis was performed, highlighting the key findings and trends across the selected studies. The clinical heterogeneity of parasagittal meningiomas, as well as variations in treatment approaches and outcomes, were thoroughly examined. This systematic review also aimed to identify gaps in the existing literature and pinpoint areas where further research is needed to improve the understanding and management of these tumors.
Data analysis
Statistical analysis was conducted using Review Manager 5.4 (Cochrane Collaboration). A random-effects model was applied to calculate the pooled mean difference (MD) in survival time between local DDS and systemic therapies. Heterogeneity among the studies was assessed using the I2 statistic to ensure the robustness of our findings. The meta-analysis results are presented in a forest plot.
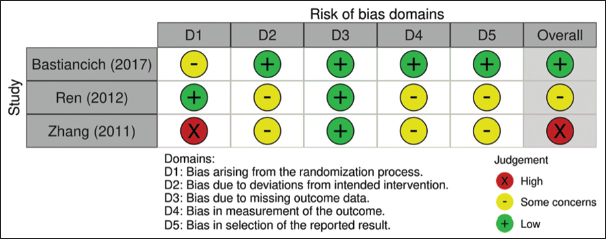
Assessment of risk of bias (ROB)
The articles included in the systematic review were assessed for ROB using the ROB-2 tool for randomized studies and the ROB in non-randomized studies of interventions (ROBINS-I) tool for non-randomized studies.[
RESULTS
Results of search strategy
Characteristics of eligible studies
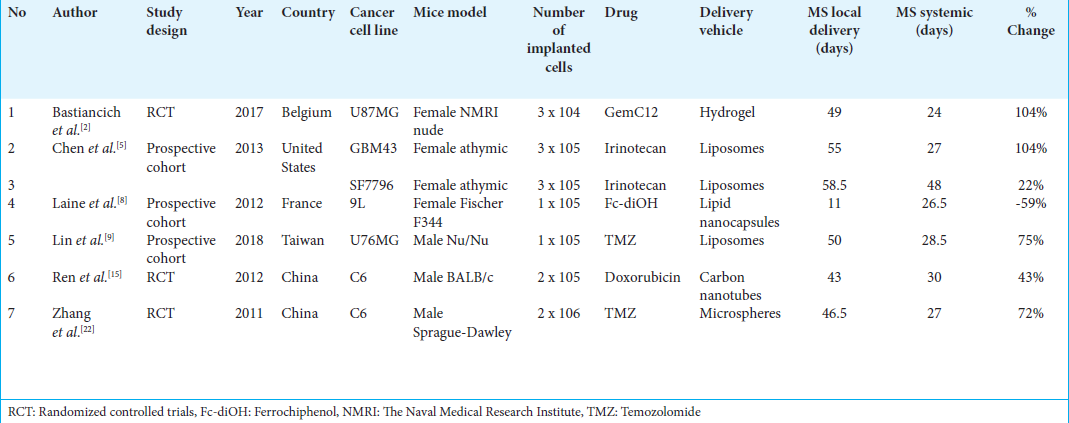
This systematic review consists of six eligible studies, including three RCTs and three prospective cohort studies [
Summary of results
Seven of the included studies compared systemic versus intra-tumoral administration of the same drug using a DDS. As outlined in
Chen et al. evaluated the antitumor efficacy of irinotecan liposomes delivered through different routes.[
Laine et al. devised an active targeting approach to deliver ferrociphenol using a cell-penetrating peptide (NFL-TBS.40-63) attached to lipid nanocapsules.[
The current standard of care for GBM involves surgical resection followed by the Stupp protocol, in which TMZ, an alkylating agent capable of crossing the BBB, is orally administered. To evaluate the potential of local delivery, Lin et al. administered TMZ-loaded liposomes directly into the tumor using CED.[
Meta-analysis
The seven eligible studies included a systemic treatment group that used the same drug as the local administration group. A meta-analysis was performed to compare the antitumor efficacy of systemic and local treatment. As depicted in
Ideally, a high I2 would warrant subgroup analyses; however, due to the limited number of studies, the authors were unable to perform these analyses, as subgroup analysis typically requires more than 10 studies. In addition, the variation in all of the variables, which are drug types, delivery vehicles, GBM cell lines, and mouse models, further complicates the feasibility of subgroup analysis.
DISCUSSION
The results of this systematic review and meta-analysis provide compelling evidence that local injection of DDS significantly improves survival in GBM models compared with systemic therapies. This finding aligns with the growing body of literature that highlights the potential of local drug delivery strategies to address the challenges of treating GBM.
The superior efficacy of local injections can be attributed to several key factors. First, by directly injecting into the tumor, the BBB can be circumvented, which is a highly selective membrane that typically prevents most drugs from entering the brain.[
Second, a review study by Rahnfeld and Luciani stated that local injection with a delivery system allows for sustained drug release, maintaining therapeutic concentrations at the tumor site over a longer period.[
Furthermore, local injection can be tailored to the specific needs of each patient, allowing for personalized treatment strategies.[
Our analysis included multiple studies that demonstrated significant improvements in MS with local injections. For instance, Chen et al. reported that local administration of Irinotecan liposomes with CED markedly improved MS compared to systemic tail vein administration, with significant results observed in the GBM43 model (P < 0.001) and the SF 7796 model (P = 0.048).[
Other notable findings include Bastiancich et al. study,[
Despite these promising results, it is important to acknowledge the limitations of the present study. The included studies were conducted using murine models, which may not fully reflect the complexity of human GBM. Further research is required to validate these findings in human clinical trials. In addition, the heterogeneity of the study designs and treatment regimens used in the included studies may have influenced the results.
Ongoing clinical trials
Ongoing clinical trials investigating local DDS for GBM are actively working to translate the encouraging results from preclinical models to clinical practice. At least three ongoing clinical trials are investigating injectable local DDS for GBM.
Adipose-derived mesenchymal stem cells (AMSCs) for Recurrent GBM
This Phase 1 trial, conducted at the Mayo Clinic in Jacksonville, Florida, investigates the safety and preliminary efficacy of locally delivered AMSCs in recurrent GBM. The trial focuses on evaluating the incidence of adverse events and assessing clinical, survival, and radiographic outcomes compared to historical controls. The local delivery of AMSCs represents a promising avenue due to its potential to bypass the BBB and provide a direct therapeutic effect at the tumor site. The use of AMSCs in GBM treatment could herald a new era of cell-based therapies tailored to recurrent disease.[
AGuIX nanoparticles with radiotherapy plus concomitant TMZ in newly diagnosed GBM (NANO-GBM)
This Phase I/II trial, conducted by the Centre Jean Perrin in France, evaluates the combination of polysiloxane Gd-chelates based nanoparticles (AGuIX) with standard radiotherapy and TMZ for NANO-GBM. AGuIX nanoparticles are designed to enhance the efficacy of radiotherapy by increasing tumor-specific radiation sensitivity while limiting damage to surrounding healthy tissue. The trial is investigating three dose levels (50 mg/kg, 75 mg/kg, and 100 mg/kg) and aims to establish the recommended dose for future studies. In addition, progression-free survival (PFS) at 6 months will be evaluated as a key endpoint, offering insight into the potential benefits of combining AGuIX with radiochemotherapy.[
NU-0129 in treating patients with recurrent GBM or gliosarcoma undergoing surgery
This Phase 0 trial is conducted at Northwestern University in Chicago, Illinois. It investigates the safety and feasibility of NU-0129, a Spherical Nucleic Acid platform that targets the Bcl2L12 gene associated with tumor growth in GBM multiforme and gliosarcoma. NU-0129 is capable of crossing the BBB and targeting tumor cells to induce apoptosis. Preliminary outcomes, including PFS and overall survival, are being assessed alongside drug concentration in serum and tumor tissue expression levels of Bcl2L12. This trial represents a significant step forward in the use of nucleic acid-based therapies for recurrent GBM.[
These ongoing trials, taking place at leading institutions across the U.S. and Europe, highlight the potential of innovative DDS and novel therapeutic strategies that aim to improve treatment efficacy and extend survival in GBM patients. Continued investigation of these therapies may bring new hope for overcoming the challenges posed by this aggressive tumor. Comparisons between preclinical findings and these ongoing human trials indicate that local DDS consistently show advantages in improving survival outcomes and enhancing drug bioavailability. However, translating these benefits into routine clinical practice requires further validation of safety, efficacy, and long-term outcomes in human subjects. The successful translation of these therapies will significantly advance the treatment landscape for GBM, offering more personalized and effective treatment options for patients.
Future directions
Future research should focus on several key areas to fully realize the potential of injectable DDS for GBM treatment. First, the development of DDS formulations capable of simultaneously delivering multiple therapeutic agents holds significant promise. Such multi DDS can target various pathways involved in GBM progression, potentially enhancing treatment efficacy.
Second, addressing the experimental variability and limitations associated with xenograft models is crucial. Although valuable, these models do not fully mimic the complexity of human GBM. Therefore, developing more representative preclinical models and standardized testing methods is essential to bridge the gap between preclinical findings and clinical applications.
Finally, conducting rigorous clinical trials to evaluate the safety, efficacy, and optimal protocols for local DDS in human patients is imperative. Standardizing testing methods across studies will facilitate more accurate comparisons and data integration, ultimately advancing the clinical translation of this promising therapeutic approach.
In summary, while local injection of DDS offers a transformative approach to GBM treatment, ongoing research, and clinical validation are necessary to overcome the existing challenges and fully leverage its potential to improve patient outcomes.
CONCLUSION
This systematic review and meta-analysis found that local injection of DDS significantly improved survival compared with systemic injections in GBM models. Injectable local drug delivery directly into GBM tumors bypasses the BBB, allowing higher concentrations of therapeutic molecules to target the tumor site effectively. This targeted approach shows great promise for overcoming the significant challenges posed by GBM treatment, including the resistance of tumor cells to conventional chemotherapy and the limitations imposed by systemic drug delivery.
Compelling evidence from various studies indicates that local DDS can enhance therapeutic efficacy and survival rates in GBM models. The ability to achieve sustained drug release at the tumor site and tailor treatments to the specific needs of each patient further underscores the potential of this approach. However, translating these promising preclinical results into clinical practice requires careful consideration of the differences between murine models and human GBM, as well as addressing the heterogeneity of study designs and treatment regimens observed in the reviewed studies.
Ongoing clinical trials are critical in evaluating the real-world effectiveness of injectable DDS in GBM patients. These trials aim to assess not only the safety and efficacy of local DDS formulations but also to identify optimal dosing regimens and combination therapies. The results from these studies will provide valuable insights into the clinical applicability of local drug delivery strategies and may pave the way for new standard treatment protocols in GBM care.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bastiancich C, Bianco J, Vanvarenberg K, Ucakar B, Joudiou N, Gallez B. Injectable nanomedicine hydrogel for local chemotherapy of glioblastoma after surgical resection. J Control Release. 2017. 264: 45-54
2. Bastiancich C, Bozzato E, Henley I, Newland B. Does local drug delivery still hold therapeutic promise for brain cancer? A systematic review. J Control Release. 2021. 337: 296-305
3. Centre Jean Perrin. AGuIX nanoparticles with radiotherapy plus concomitant temozolomide in the treatment of newly diagnosed glioblastoma (NANO-GBM). Available from: https://clinicaltrials.gov/study/NCT04881032?cond=glioblastoma&intr=nanoparticle&rank=1 [Last accessed on 2024 Sep 06].
4. Cha GD, Jung S, Choi SH, Kim DH. Local drug delivery strategies for glioblastoma treatment. Brain Tumor Res Treat. 2022. 10: 151-7
5. Chen PY, Ozawa T, Drummond DC, Kalra A, Fitzgerald JB, Kirpotin DB. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013. 15: 189-97
6. Dobes M, Shadbolt B, Khurana VG, Jain S, Smith SF, Smee RI. A Multicentre study of primary brain tumor incidence in Australia (2000-2008). Neuro Oncol. 2011. 13: 783-90
7. Hersh AM, Alomari S, Tyler BM. Crossing the blood-brain barrier: Advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mol Sci. 2022. 23: 4153
8. Laine AL, Huynh NT, Clavreul A, Balzeau J, Béjaud J, Vessieres A. Brain tumor targeting strategies via coated ferrociphenol lipid nanocapsules. Eur J Pharm Biopharm. 2012. 81: 690-3
9. Lin CY, Li RJ, Huang CY, Wei KC, Chen PY. Controlled release of liposome-encapsulated temozolimide for brain tumor treatment by convenction-enhanced delivery. J Drug Target. 2018. 26: 325-32
10. Mayo Clinic. Clinical trials: Adipose-derived mesenchymal stem cells (AMSCs) for recurrent glioblastoma. Available from: https://www.mayo.edu/research/clinical-trials/cls-20545768 [Last accessed on 2024 Sep 06].
11. Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
12. Northwestern University. NU-0129 in treating patients with recurrent glioblastoma or gliosarcoma undergoing surgery. Available from: https://clinicaltrials.gov/study/NCT03020017?cond=glioblastoma&intr=nanoparticle&rank=3 [Last accessed on 2024 Sep 06].
13. Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel wafers in the treatment of malignant glioma: A systematic review. Curr Oncol. 2007. 14: 189-94
14. Rahnfeld L, Luciani P. Injectable lipid-based depot formulations: Where do we stand?. Pharmaceutics. 2020. 12: 567
15. Ren JF, Shen S, Wang D, Xi Z, Guo L, Pang Z. The targeted delivery of anticancer drugs to brain by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012. 33: 3324-33
16. Sterne JA, Hernán M, Reeves B, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 355: i4919
17. Sterne JA, Savocić J, Page MJ, Elbers PG, Blencowe NS, Boutron I. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ. 2019. 366: i4898
18. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
19. Urhie O, Turner R, Lucke-Wold B, Radwan W, Ahn J, Gyure K. Glioblastoma survival outcomes at a tertiary hospital in Appalachia: Factors impacting the survival of patients following implementation of the Stupp Protocol. World Neurosurg. 2018. 115: 59-66
20. Van Solinge TS, Nieland L, Chiocca EA, Broekman ML. Advances in local therapy for glioblastoma -taking the fight to the tumor. Nat Rev Neurol. 2022. 18: 221-36
21. Yu F, Asghar S, Zhang M, Zhang J, Ping Q, Xiao Y. Local strategies and delivery systems for the treatment of malignant gliomas. J Drug Target. 2018. 27: 367-78
22. Zhang YH, Zhang H, Liu JM, Yue ZJ. Temozolimide/PLGA microparticles: A new protocol for treatments of glioma in rats. Med Oncol. 2011. 28: 901-6
23. Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019. 25: 462-9