- Department Neurosurgery, Osaka Police Hospital, Osaka, Japan.
DOI:10.25259/SNI_239_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shuta Aketa, Daisuke Wajima, Masayoshi Kiyomoto, Natsuhiko Izumi, Taiji Yonezawa. Optimum concentration of iodine contrast agent injection for best stent visualization using Neuroform Atlas stent during stent-assisted coil embolization: Case reviews based on in vitro experiments. 27-Jun-2020;11:170
How to cite this URL: Shuta Aketa, Daisuke Wajima, Masayoshi Kiyomoto, Natsuhiko Izumi, Taiji Yonezawa. Optimum concentration of iodine contrast agent injection for best stent visualization using Neuroform Atlas stent during stent-assisted coil embolization: Case reviews based on in vitro experiments. 27-Jun-2020;11:170. Available from: https://surgicalneurologyint.com/surgicalint-articles/10106/
Abstract
Background: The present study aimed to evaluate the influence of contrast agent concentration (Conc) on the visibility of Neuroform Atlas in vitro and in clinical cases.
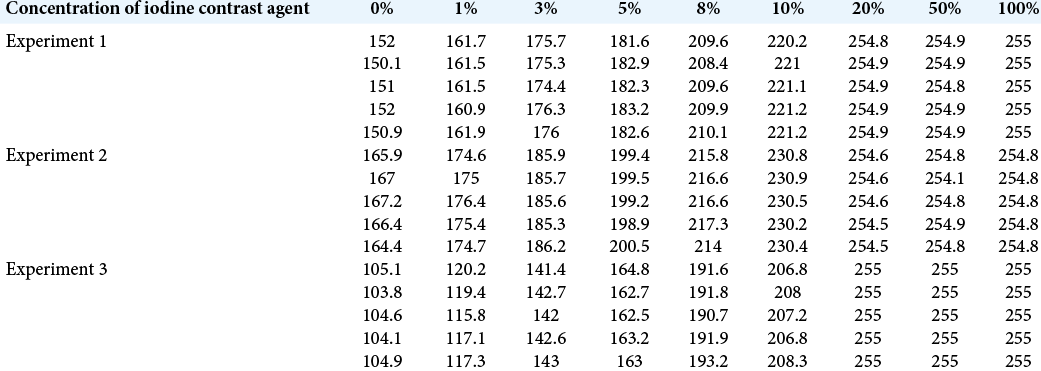
Methods: A plastic tube was filled with several Conc. in saline (experiment 1) and blood (experiment 2). Thereafter, the Neuroform Atlas was placed around the plastic tube in an acrylic shield case. In experiment 3, the Neuroform Atlas was placed in the internal carotid artery of the endo vascular evaluator endovascular training system with an injection of several Conc in saline. Five slices of the axial images obtained using the 3D-cone-beam computed tomography (3D-CBCT) with the digital subtraction angiography system were evaluated. A 1-cm2 circular center, which showed the contrast agent in saline or blood, was determined as the region of interest, and its pixels were evaluated.
Results: Radiation density (Rd) was directly proportional to the contrast agent in saline and blood (experiment 1: (Rd (pixel)) = 6.8495 × (concentration (%)) + 152.72 (R2 = 0.99), experiment 2: (Rd (pixel)) = 6.2485 × (concentration (%)) + 167.42 (R2 = 0.9966), experiment 3: (Rd (pixel)) = 10.287 × (concentration (%)) + 108.26 (R2 = 0.993)]. Rd calculated similarly in our cases (concentration varied from 5% to 8%) was between the range of “Rd of experiment 2” and “Rd of experiment 3.”
Conclusion: Based on our in vitro experiments, with 5–8% concentration, Neuroform Atlas stent deployment with complete neck coverage by the bulging stent and wall apposition was visualized on 3D-CBCT.
Keywords: Coil embolization, Iodine contrast, Neuroform Atlas stent, Optimum, Stent visualization
INTRODUCTION
Stent-assisted coil embolization is an effective neuro-interventional method for wide-necked aneurysms. Neuroform Atlas stents (Stryker, Fremont, CA, USA) can be delivered through the “coil catheter,” and their safety and efficacy, ease of delivery, and appropriate opening and deployment have been the subjects of recent reports.[
However, this stent is considered less visible during the procedure due to its small metal attachments, except the stent markers at each end. Verifying the optimal arterial wall apposition to the parent artery is important during the procedure, especially when applied to the bulging technique in patients with wide-necked aneurysms.
In this study, we aimed to report the results of our in vitro experiments and based on these experiments, the clinical characteristics of the patients with wide-necked aneurysms treated with Neuroform Atlas stent-assisted coiling. The stent bulging technique using the 3D-cone-beam computed tomography image (3D-CBCT) with Innova IGS 630 digital subtraction angiography (DSA) system (GE Healthcare, Chicago, IL, USA) was used to confirm the neck coverage of the stent.
MATERIALS AND METHODS
In vitro experiments
All procedures and projects were approved by the review board of our hospital. In addition, informed consent and approval were obtained from the patients included in our study.
Experiment 1: Radiation density (Rd) changes with change in the concentration of iodine contrast agent in the saline static state
Six plastic tubes, 3.5 mm in diameter, were filled with varied concentrations of iodine contrast agent in saline (0%, 1%, 3%, 5%, 8%, and 10%). Thereafter, a 4 mm×21 mm Neuroform Atlas stent was placed around the plastic tube in an acrylic shield case [
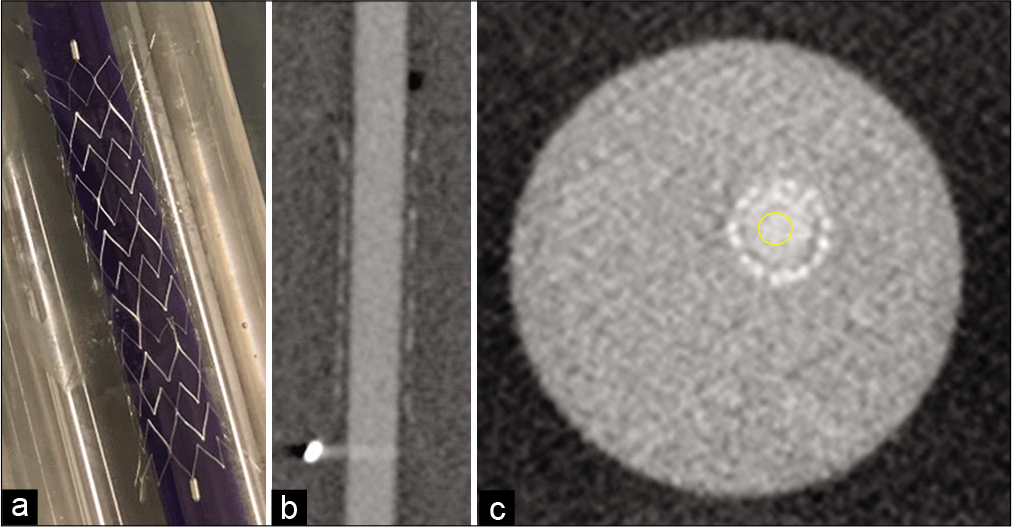
Figure 1:
In vitro experiment. In experiments 1 and 2, a plastic tube, 3.5 mm in diameter, was filled with varied concentrations of iodine contrast agent in saline and blood (0%, 1%, 3%, 5%, 8%, and 10%). Thereafter, a 4 mm×21 mm Neuroform Atlas stent was put around the plastic tube in the acrylic shield case (a). Of the 3D-cone-beam computed tomography (3D-CBCT) images obtained using Innova IGS 630 digital subtraction angiography system (b), five slices of the axial plane were evaluated using the Image J software. A circular (1 cm2) area in the center showed the iodine contrast agent in saline and was determined as the region of interest (ROI) (c). The pixels of those ROIs were evaluated. In experiment 3, a 4 mm×21 mm Neuroform Atlas stent was replaced in the internal carotid artery of the EVE endovascular training system (FAIN-Biomedical Inc., California, USA). Varied concentrations of the iodine contrast agent in saline (0%, 1%, 3%, 5%, 8%, and 10%) were injected from the pump (injection speed of 5 ml/sec for 16 s, 3 s for radiographic delay, 3D-CBCT for 13 s).
Of the 3D-CBCT images obtained using the Innova IGS 630 DSA system [
Experiment 2: Rd changes with change in the concentration of iodine contrast agent in blood static state
Six plastic tubes, 3.5 mm in diameter, were filled with varied concentrations of the iodine contrast agent in the arterial blood of a healthy volunteer (0%, 1%, 3%, 5%, 8%, and 10%). Thereafter, a 4 mm×21 mm Neuroform Atlas stent was placed around the plastic tube in an acrylic shield case.
Of the 3D-CBCT images obtained using the Innova IGS 630 DSA system [
Experiment 3: Rd changes with change in the concentration of iodine contrast agent in saline flow state
A 4 mm × 21 mm Neuroform Atlas stent was replaced in the internal carotid artery (ICA) of the endo vascular evaluator (EVE) endovascular training system (FAIN-Biomedical Inc., California, USA). Varied concentrations of the iodine contrast agent in saline (0%, 1%, 3%, 5%, 8%, and 10%) were injected from the pump (the injection speed was 5 ml/sec for 16 s, 3 s for radiographic delay, 3D-CBCT for 13 s).
Of the 3D-CBCT images obtained using the Innova IGS 630 DSA system [
Statistical analysis
Multiple regression analysis was performed to analyze the association with each experiment in vitro (experiments 1–3). The two-sample t-test was used to compare the Rd (pixel) according to 5% and 8% iodine contrast agent in vitro and for the clinical cases. Statistical significance was defined as P < 0.05. All statistical analyses were performed using the Statview Version 5.0 (SAS Institute, North Carolina, USA) and Microsoft Excel Version 2016 (Microsoft, USA).
RESULTS
Results of in vitro experiments 1–3
Rd (pixel) data in experiments 1–3 are shown in [
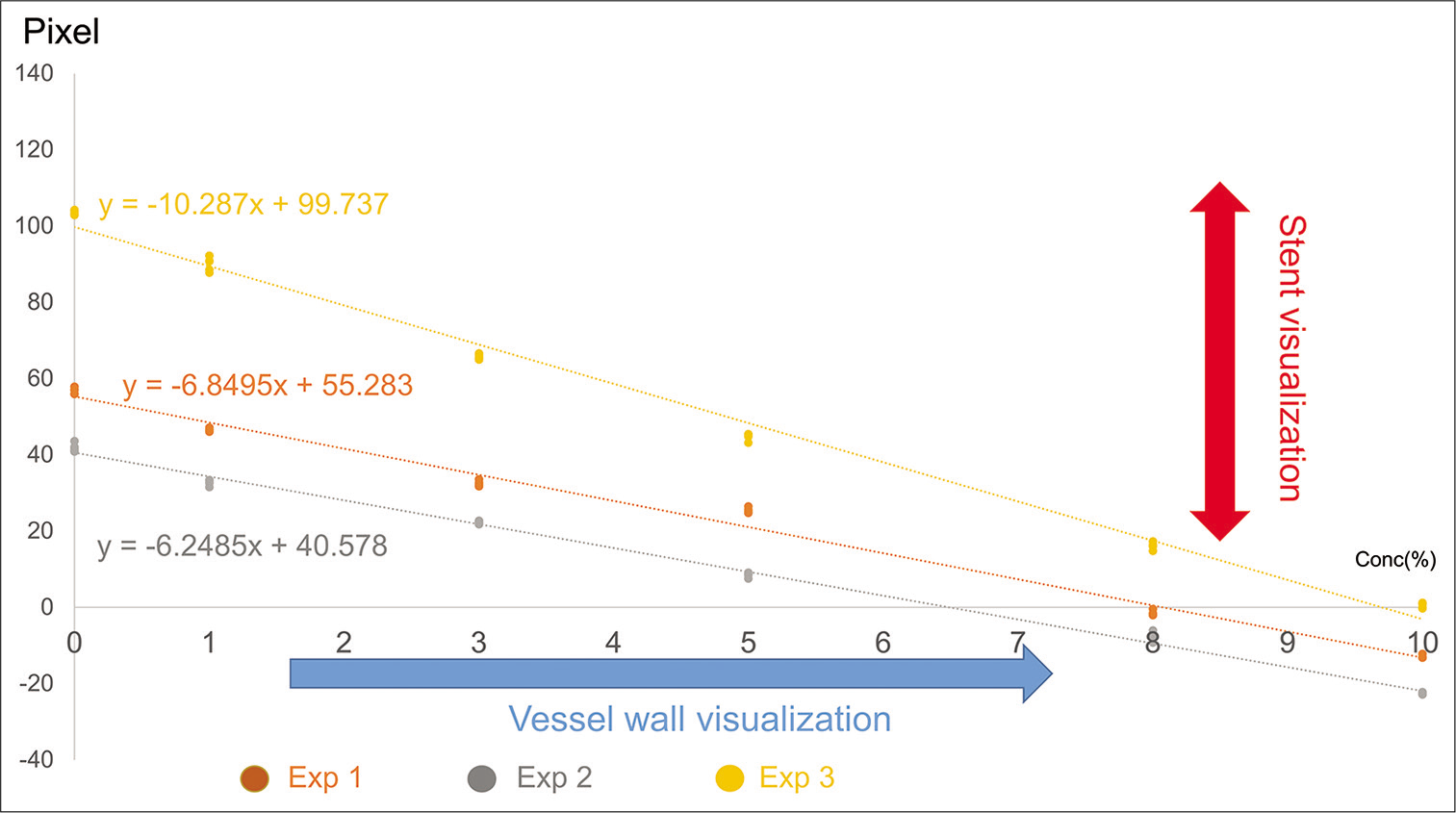
Figure 2:
Evaluation of the results of experiments 1–3 and radiation density of the Neuroform Atlas stent strut. The radiation density of the Neuroform Atlas stent strut calculated with the Image J software varies from 208 to 256 pixels. ((Maximum radiation density of the Neuroform Atlas strut (256 pixel)) – [Radiation density of iodine contrast agent in the blood or saline]) was calculated and graphed ((Radiation density of experiment 1 (pixel)) = −6.8495 × (Concentration of iodine contrast agent (%)) + 55.283 (R2=0.99); (Radiation density of experiment 2 (pixel)) = −6.2485 × (Concentration of iodine contrast agent (%)) + 40.578 (R2=0.9966); (Radiation density of experiment 2 (pixel)) = −10.287 × (Concentration of iodine contrast agent (%)) + 99.737 (R2=0.993)).
Experiment 1: The Rd was directly proportional to the concentration of the iodine contrast agent in saline ((Rd (pixel)) = 6.8495 × (concentration of iodine contrast agent (%)) + 152.72 (R2=0.99)).
Experiment 2: The Rd was directly proportional to the concentration of the iodine contrast agent in blood ((Rd (pixel)) = 6.2485 × (concentration of iodine contrast agent (%)) + 167.42 (R2=0.9966)).
Experiment 3: The Rd was directly proportional to the concentration of the iodine contrast agent in saline flow state ((Rd (pixel)) = 10.287 × (concentration of iodine contrast agent (%)) + 108.26 (R2=0.993)).
The Rd of Neuroform Atlas stent strut calculated with the Image J software varied from 208 to 256 pixels in our study ((Maximum Rd of Neuroform Atlas strut (256 pixel)) − (Rd of iodine contrast agent in blood or saline)) was calculated and graphed ((Rd of experiment 1 (pixel)) = −6.8495 × (Concentration of iodine contrast agent (%)) + 55.283 (R2=0.99); (Rd of experiment 2 (pixel)) = −6.2485 × (Concentration of iodine contrast agent (%)) + 40.578 (R2=0.9966); and (Rd of experiment 2 (pixel)) = −10.287 × (Concentration of iodine contrast agent (%)) + 99.737 (R2=0.993)) [
Results of the in vitro experiments and clinical cases
We studied 18 cases (iodine contrast agent concentration varied from 5% to 8%) and examined five slices each of the axial planes of that image using the Image J software. A circular (1 cm2) area in the center of the iodine contrast agent was measured as ROI. All these Rd values were between the range of “Rd of experiment 2” and “Rd of experiment 3” [
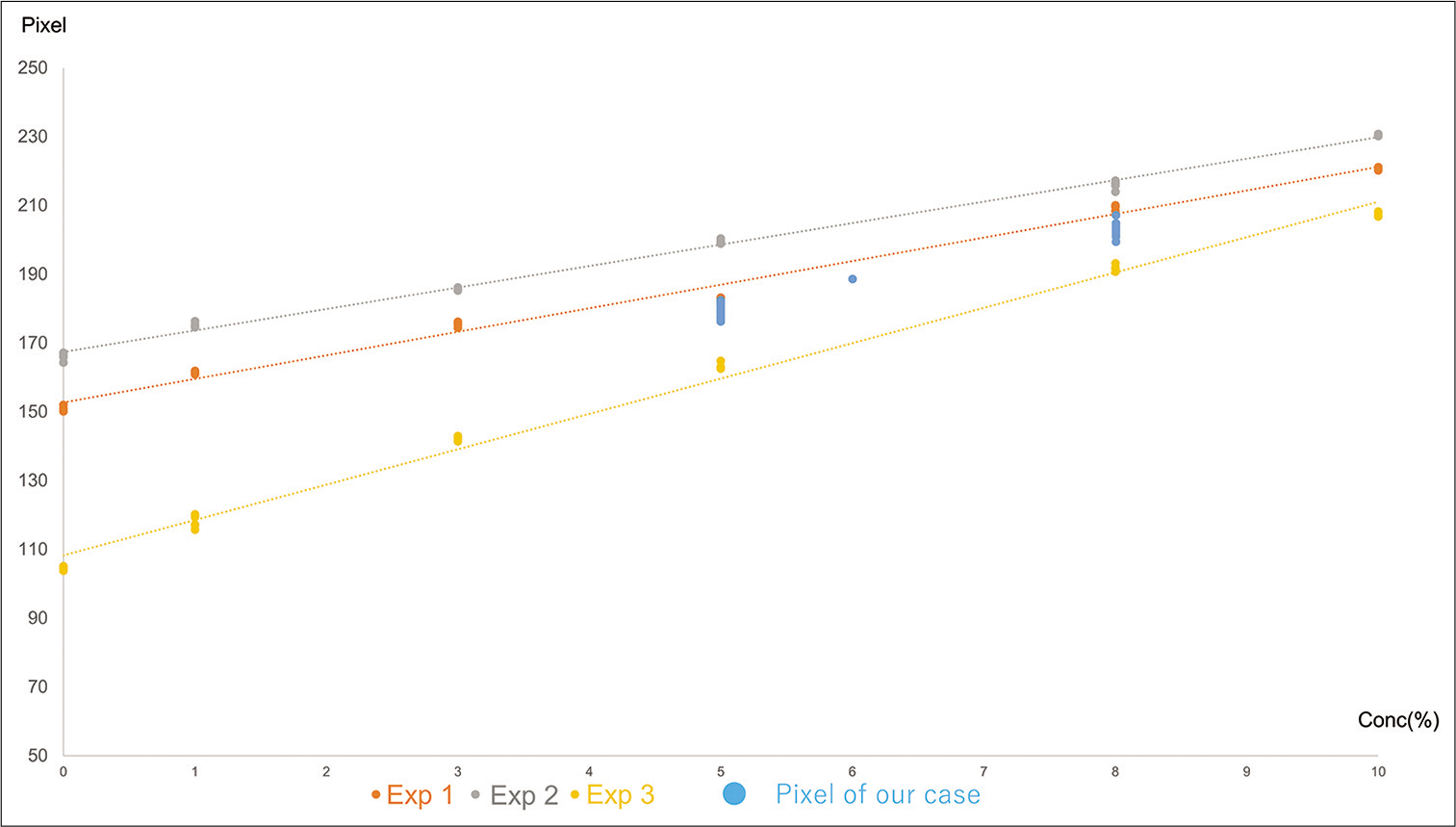
Figure 3:
Results of the in vitro experiments and our clinical cases. We experienced 18 cases (iodine contrast agent concentration varies 5–8%), and five slices of the axial plane of that image are evaluated by Image J software. A circular area (1 cm2) in the center of iodine contrast agent is measured as region of interest. All these radiation density values range from “Radiation density of experiment 2” and “Radiation density of experiment 3.” The radiation density (pixel) of 5% iodine contrast agent concentration cases (10 cases) (169.33 ± 2.345) is significantly superior to those of experiment 3 (162.85 ± 0.284, P = 0.0131) and significantly inferior to those of experiment 2 (199.53 ± 0.666, P = 0.0002). The radiation density (pixel) of 8% iodine contrast agent concentration cases (7 cases) (200.86 ± 3.289) is significantly superior to those of experiment 3 (191.93 ± 1.011, P = 0.0235) and significantly inferior to those of experiment 2 (216.11 ± 1.421, P = 0.0005).
The 18 cases are summarized in [
The Rd (pixel) of the cases with 5% concentration of the iodine contrast agent in experiment 1 (10 cases) (169.33 ± 2.345) was significantly superior to that of experiment 3 (162.85 ± 0.284, P = 0.0131) and significantly inferior to that of experiment 2 (199.53 ± 0.666, P = 0.0002). The Rd (pixel) of 8% iodine contrast agent concentration cases (7 cases) (200.86 ± 3.289) in experiment 1 was significantly superior to that of experiment 3 (191.93 ± 1.011, P = 0.0235) and significantly inferior to that of experiment 2 (216.11 ± 1.421, P = 0.0005).
Representative case
Case 1
A 73-year-old woman was diagnosed with multiple unruptured aneurysms of the anterior communicating artery (A-com A), left basilar artery (BA), bifurcation of the superior cerebellar artery (SCA), and tip of the BA underwent coiling for BA aneurysms after clipping of the A-com A aneurysm [
Figure 4:
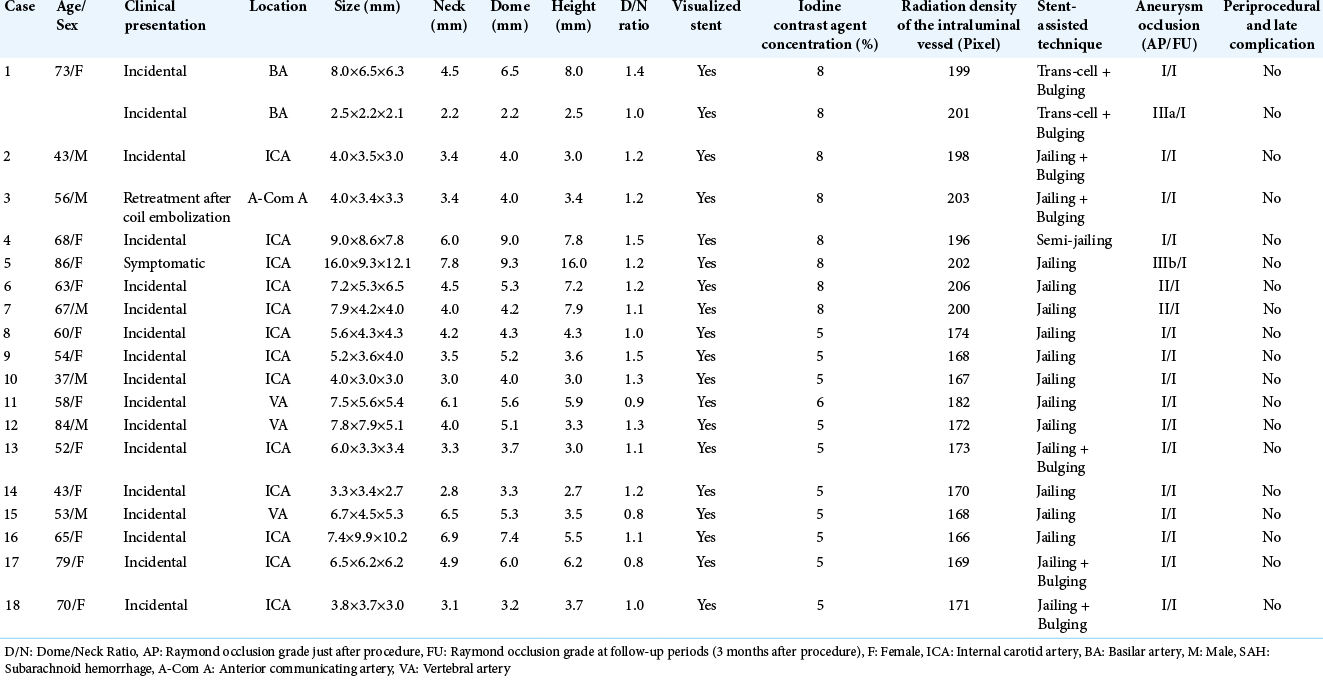
Representative Case 1. A 73-year-old woman was diagnosed with two unruptured aneurysms of the left basilar artery (BA) – superior cerebellar artery (SCA) bifurcation portion and the BA tip portion (a: Right vertebral artery angiography [Rt VAG]). Stent-assisted coil embolization was performed. A 4 mm×21 mm Neuroform Atlas stent was placed using the bulging technique, between the left PCA and BA trunk portion, covering the necks of both the left BA-SCA and BA tip aneurysms. After confirming the good stent bulging, effective covering of both aneurysmal necks and vessel wall apposition was seen on 3D-cone-beam computed tomography (3D-CBCT) (b: 3D-CBCT). Coiling both aneurysms was successfully performed according to the trans-cell method (c: Rt VAG, d: 3D-CBCT). Follow-up DSA at 6 months after the stent-assisted coil embolization showed complete occlusion of both aneurysms and good blood flow on stent placement (e: Rt VAG).
Under general anesthesia and systemic heparinization, a 6 French (Fr) Roadmaster guiding catheter (Goodman, Nagoya, Aichi, Japan) was introduced into the right vertebral artery (VA) through the right femoral artery, and a 5 Fr Simmonds-shaped diagnostic catheter was introduced into the left VA through the left femoral artery. Through the guiding catheter, an Excelsior SL-10 Microcatheter (Stryker, Fremont, CA, USA) was navigated into the left PCA P1 over a CHIKAI 0.014 inch microwire (Asahi Intec, Nagoya, Aichi, Japan) for stent delivery, and a 4 mm×21 mm Neuroform Atlas stent was deployed using the bulging technique from the left PCA to the trunk portion of the BA across the necks of both left BA-SCA and BA tip aneurysms. The microcatheter that was used for stent delivery was advanced through the stent into the left BA-SCA aneurysm and used for coiling. After confirming optimal stent bulging and effective covering of the aneurysmal necks and vessel wall apposition on 3D-CBCT with 8% diluted contrast media [
Case 2
A 43-year-old male patient presented with a growing unruptured aneurysm of the left ICA at the bifurcation of the ophthalmic artery (Oph A) [
Figure 5:
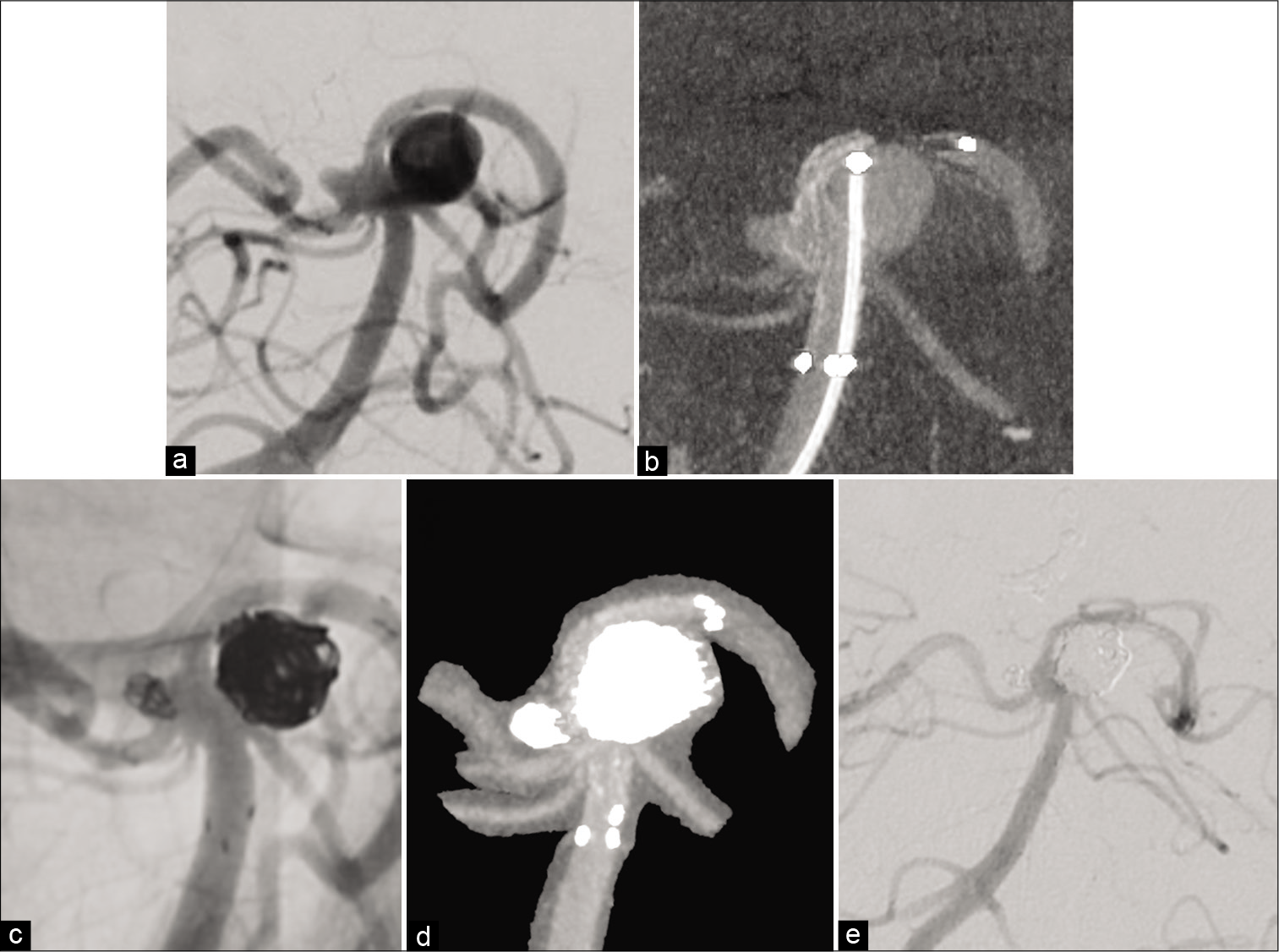
Representative Case 2. A 43-year-old male patient was diagnosed with an unruptured aneurysm of the left internal carotid artery–ophthalmic artery (Oph A) bifurcation portion (a: left carotid artery angiography (Lt CAG), b: Lt CAG translucent image). Stent-assisted coil embolization was performed. A 4.5 mm×21 mm Neuroform Atlas stent was deployed to protect the origin of the Oph A using the bulging technique. After confirming stent deployment with good herniation of the stent struts into the aneurysmal neck and the origin of Oph A and vessel wall apposition on 3D-CBCT (c: 3D-CBCT), coil embolization was performed as per the jailing technique (d: Lt CAG, e: 3D-digital subtraction angiography fusion image).
Under general anesthesia and systemic heparinization, a 6 Fr Axcelguide guiding sheath (Medikit, Tokyo, Japan) was introduced into the origin of the left ICA through the right femoral artery, and coaxially, a 6 Fr Cerulean catheter DD6 (Medikit) was introduced into the petrous part of the left ICA. First, balloon-assisted coiling was attempted, but we could not complete coil embolization due to the framing coil prolapse after deflation of the balloon. Therefore, stent- assisted coiling was performed continuously. After changing the balloon catheter to an Excelsior SL-10 Microcatheter in the left ICA, a 4.5 mm×21 mm Neuroform Atlas stent was selected according to the diameter measuring 4.5 mm and deployed with the bulging stent to protect the origin of the Oph A. After confirming the stent deployment with optimal herniation of the stent struts into the aneurysmal neck and the origin of Oph A and vessel wall apposition on 3D-CBCT with 8% diluted contrast media [
DISCUSSION
It is particularly important to perform stent-assisted coil embolization for wide-necked aneurysms to provide easy delivery of the systems, good wall apposition to the parent artery, and visualization of the stent strut during the procedure.[
The Neuroform Atlas stent is a modified open-cell stent that has recently been introduced for endovascular treatment of wide-necked aneurysms and is compatible with the current microcatheters that have an internal diameter of 0.0165''. This design improves delivery in small and/or tortuous vessels and is applicable to the trans-cell method used in small or distal aneurysms. The stent strut comprises zigzag shapes that are joined by four interconnections evenly spaced throughout the strut. Each alternating strut is made up of 8 or 12 crowns, with the most proximal row of cells being a closed cell of 12 crowns. This new design was perhaps the reason for more accurate stent apposition in the carotid siphon. These observations suggest that low-profile open-cell stents tend to fit adequately onto the vessel wall compared with other stents.
On the contrary, the Neuroform Atlas stent is less visible due to its small metal attachments. The visibility is essential to allow accurate placement and deployment of the device. Our experiments demonstrated that Rd was directly proportional to the concentration of the iodine contrast agent in saline and blood both in static and flow states. In addition, the difference between the Rd of the Neuroform Atlas stent strut and that of the iodine contrast agent in saline and blood in static and flow states is proportional to the visibility and contrast of the stent strut and arterial wall. The least concentration for arterial wall visibility in 3D-CBCT was approximately 5%, and contrast of the stent strut and arterial wall was below 8% from our in vitro experiments.
Based on the result of our in vitro experiments, 5–8% dilution of the contrast media is the valid range for the visibility of the stent strut. We confirmed in our data analysis that the use of this dilution of contrast media was effective for the visibility and contrast of the stent strut and arterial wall during stent- assisted coil embolization.
In all our series, complete occlusion of wide-necked aneurysms was achieved with the bulging technique using the Neuroform Atlas stent. In our cases, no periprocedural complications occurred, including intervention-related and thromboembolic events. Gross et al. reported rates of initial complete angiographic obliteration (Raymond 1), and the follow-up obliteration (Raymond 1 or 2) class was significantly greater for aneurysms treated with the Atlas stent than those treated with low-profile visualized intraluminal support (LVIS) (Terumo, Tokyo, Japan) or LVIS Jr.[
Visualization of the Neuroform Atlas stent by 3D-CBCT in our cases was safe and assured using stent-assisted coiling with stent bulging technique for wide-necked aneurysms. The bulging technique with the Neuroform Atlas stent meant the simple deployment of the stent selected according to the diameter to cover the neck of the aneurysm with the bulging stent. The procedure comprised opening the stent, maintaining the position of the delivery wire on the midline of the vessel without stent push. Our experience demonstrated that the Neuroform Atlas stent-assisted coiling was a safe and feasible technique to treat wide-necked aneurysms in accordance with recent reports of their safety and efficacy.[
Limitations
Our study has several limitations. Only a small number of cases from a single center were included in the study. Not all patients who underwent stent-assisted embolization could be analyzed as the imaging protocol for stent apposition confirmation was not standardized in our institution. This may have introduced selection bias.
Furthermore, we did not check coil mass interfere visualization of the stent due to metal artifact in our experiment. We performed our experiments without coil mass in cerebral aneurysm. It is because that first, the stent apposition confirmation is confirmed by 3D-CBCT without coil mass in cerebral aneurysm, after that, we performed our stent-assisted coil embolization technique.
In addition, we investigated stent apposition for only one type of stent and did not assess stent apposition of flow diverters. Similarly, the reconstruction parameters were not standardized and could potentially affect the visibility of the stent structures and vessels. Finally, the radiation dose administered to the patients was not investigated.
CONCLUSION
In this study, we reported our experiences of successful visualization of the deployed Neuroform Atlas stent using the 3D-CBCT image with 5–8% dilution of the iodine contrast agent using the Innova IGS 630 biplane angiography system along with successful Neuroform Atlas stent-assisted coiling with the bulging technique for wide-neck aneurysms. Thus, the Neuroform Atlas stent-assisted coiling with the bulging technique under visible confirmation of the deployed stent using the 3D-CBCT image is a safe and feasible way to treat wide-necked intracranial aneurysms.
Declaration of patient consent
Patient’s consent not obtained as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cay F, Peker A, Arat A. Stent-assisted coiling of cerebral aneurysms with the neuroform Atlas stent. Intervent Neuroradiol. 2018. 24: 263-9
2. Cho YD, Sohn CH, Kang HS, Kim JE, Cho WS, Hwang G. Coil embolization of intracranial saccular aneurysms using the Low-profile visualized intraluminal support (LVIS™) device. Neuroradiology. 2014. 56: 543-51
3. Gross BA, Ares WJ, Ducruet AF, Jadhav AP, Jovin TG, Jankowitz BT. A clinical comparison of Atlas and LVIS Jr stent-assisted aneurysm coiling. J Neurointerv Surg. 2019. 11: 171-4
4. Grossberg JA, Hanel RA, Dabus G, Keigher K, Haussen DC, Sauvageau E. Treatment of wide-necked aneurysms with the low-profile visualized intraluminal support (LVIS Jr) device: A multicenter experience. J Neurointervent Surg. 2017. 9: 1098-102
5. Heller RS, Miele WR, Do-Dai DD, Malek AM. Crescent sign on magnetic resonance angiography revealing incomplete stent apposition: Correlation with diffusion weighted changes in stent-mediated coil embolization of aneurysms. J Neurosurg. 2011. 115: 624-32
6. Turner RD, Turk A, Chaudry I. Low-profile visible intraluminal support device: Immediate outcome of the first three US cases. J Neurointervent Surg. 2013. 5: 157-60
7. Ulfert C, Pham M, Sonnberger M, Amaya F, Trenkler J, Bendszus M. The Neuroform Atlas stent to assist coil embolization of intracranial aneurysms: A multicentre experience. J Neurointervent Surg. 2018. 10: 1192-
8. Wang J, Vargas J, Spiotta A, Chaudry I, Turner RD, Lena J. Stent-assisted coiling of cerebral aneurysms: A single-center clinical and angiographic analysis. J Neurointervent Surg. 2018. 10: 687-92