- Department of Neurosurgery, Harasanshin Hospital, Hakata-ku, Fukuoka, Japan.
- Department of Pathology, Harasanshin Hospital, Hakata-ku, Fukuoka, Japan.
Correspondence Address:
Yusuke Funakoshi
Department of Pathology, Harasanshin Hospital, Hakata-ku, Fukuoka, Japan.
DOI:10.25259/SNI_857_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yusuke Funakoshi1, Tadahisa Shono1, Ai Kurogi1, Shinji Kono2. Osteosarcoma of the temporal bone occurring 40 years after radiotherapy: A technical case report presenting en bloc resection of intra- and extracranial lesions followed by a one-stage reconstruction. 14-Apr-2021;12:152
How to cite this URL: Yusuke Funakoshi1, Tadahisa Shono1, Ai Kurogi1, Shinji Kono2. Osteosarcoma of the temporal bone occurring 40 years after radiotherapy: A technical case report presenting en bloc resection of intra- and extracranial lesions followed by a one-stage reconstruction. 14-Apr-2021;12:152. Available from: https://surgicalneurologyint.com/surgicalint-articles/10718/
Abstract
Background: Osteosarcoma (OS) is a malignant tumor of the bone, which rarely occurs in the head-and-neck regions as a primary or a secondary malignancy. Adequate surgical resection is currently the mainstay of treatment for head-and-neck OS; however, en bloc resection and reconstruction can be difficult because the anatomies of these regions are complex. We present a case of an OS arising from the temporal bone 40 years after radiation therapy, which was successfully treated with en bloc resection and a one-stage reconstruction using intraoperative tissue expansion technique.
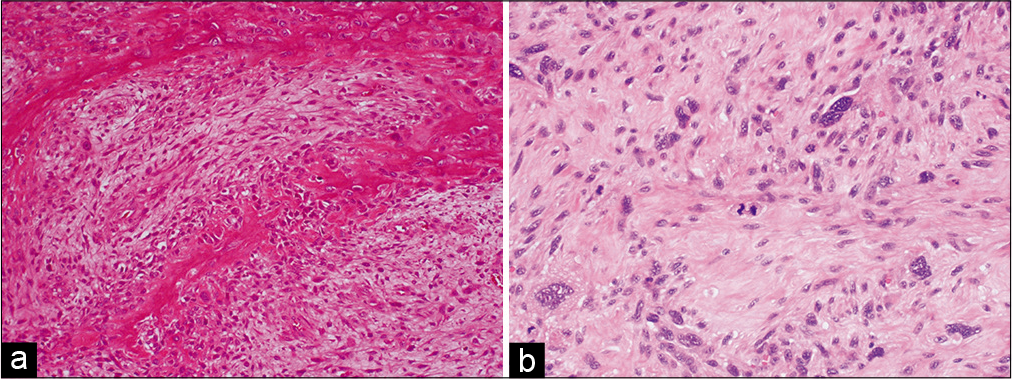
Case Description: A 62-year-old woman who underwent surgery and radiotherapy for a left temporal lesion 40 years before presentation was hospitalized for aphasia and a right hemiparesis. She had a 4 × 3 cm subcutaneous mass in the left temporal area of the head. Computed tomography imaging showed destruction of the left temporal bone and a partially calcified mass. Magnetic resonance imaging showed an enhancing mass with intracranial and extracranial cystic components (5 cm and 3 cm in diameter, respectively). Due to rapid growth of the lesion, a semi-urgent surgery was performed. In this operation, a continuous narrow craniectomy was performed around the tumor using a ruler. Then, en bloc resection of the tumor, with adjacent skin, temporal muscle, skull, dura mater, and cerebral cortex, was achieved. Subsequently, a one-stage reconstruction of the dura mater, skull, and skin of the head was performed using fascia lata, artificial bone, and a local skin flap combined with intraoperative tissue expansion using a 20-French Foley catheter. Postoperative histological examinations revealed the tumor to be an OS.
Conclusion: We have presented a rare case of an OS occurring from the temporal bone 40 years after radiation therapy. We describe our experience and the surgical methods in this case to provide options for surgical strategies in patients with head-and-neck OS.
Keywords: En bloc resection, Osteosarcoma, Radiation, Reconstruction, Temporal bone
INTRODUCTION
Osteosarcoma (OS) is a malignant bone tumor that can occur anywhere in the body, though most commonly affects the long bones in young adults and children. The incidence of OS is estimated to be 1:100,000 per year,[
CASE DESCRIPTION
Initial presentation
A 62-year-old woman who underwent surgery and radiation therapy for a left temporal lesion at another hospital 40 years before presentation was admitted to our hospital due to progressive aphasia and right hemiparesis. She was found to have a 4 × 3 cm subcutaneous mass in the left temporal area of the head [
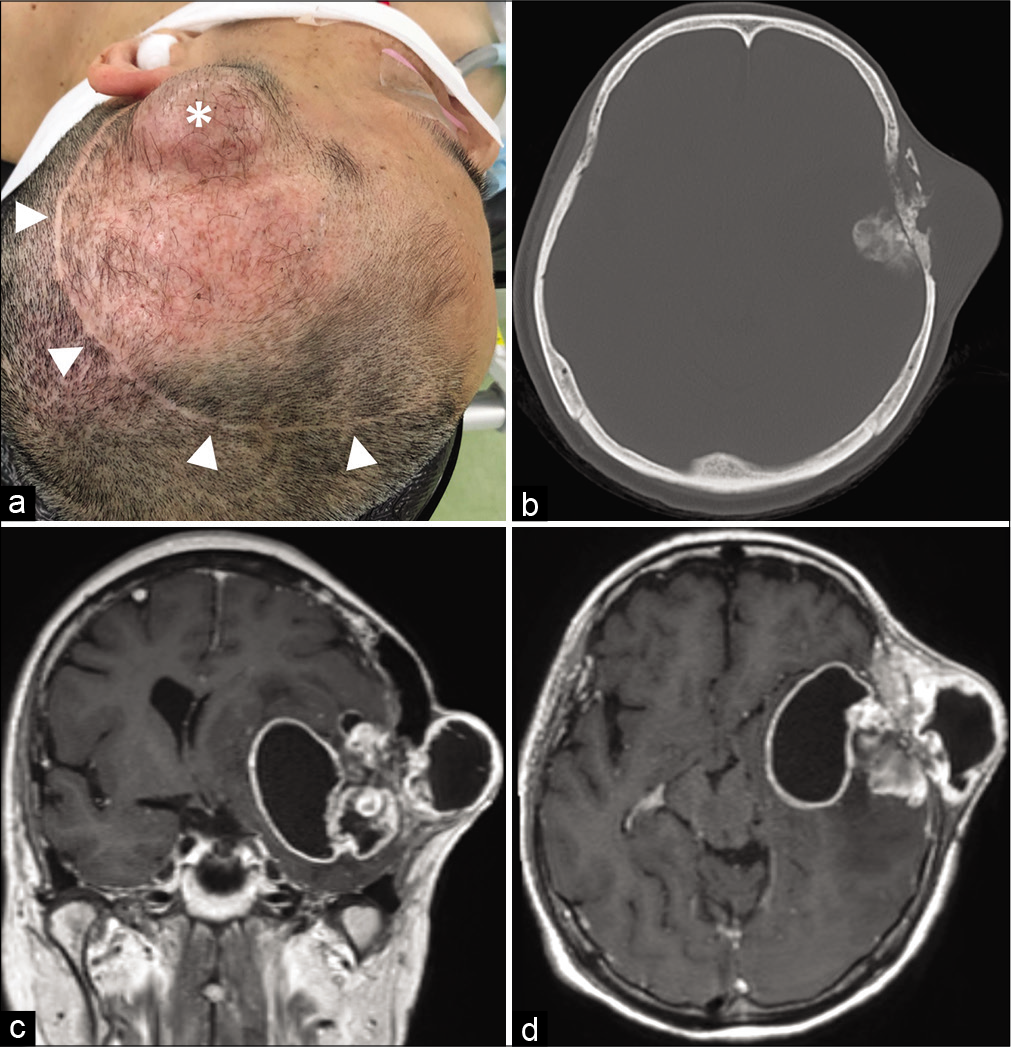
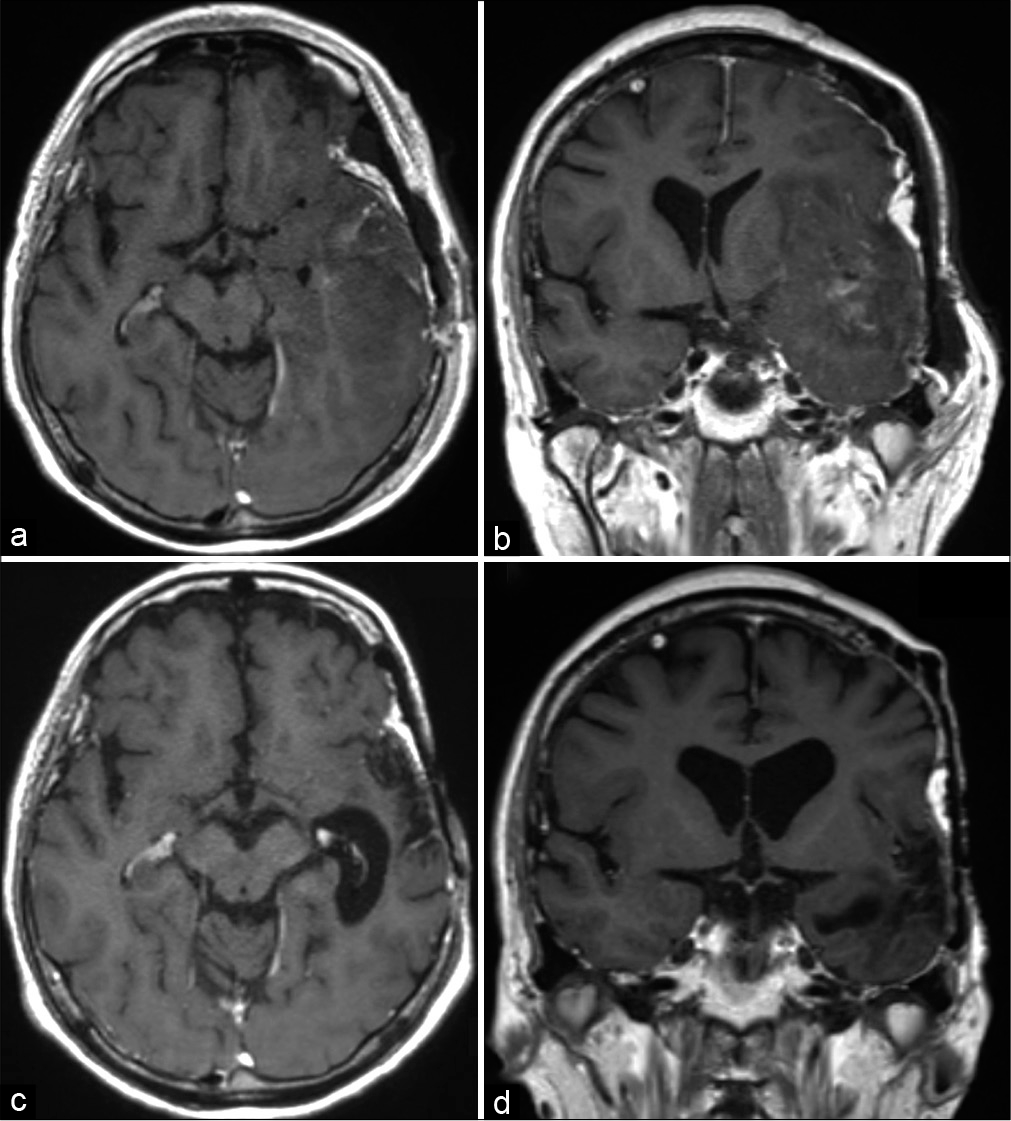
Figure 1:
Clinical/radiologic findings at patient presentation. (a) Photograph showing a 4 × 3 cm subcutaneous mass in the left temporal area of the head (white asterisk) and a previous skin incision (white arrowheads). (b) Computed tomography imaging showing destruction of the left temporal bone and a partially calcified mass lesion. (c and d) T1-weighted contrast-enhanced magnetic resonance imaging showing an enhancing mass with intracranial and extracranial cystic components.
Operative procedure
The surgical procedure was performed in the right semilateral position under general anesthesia. The scar of the previous operation [
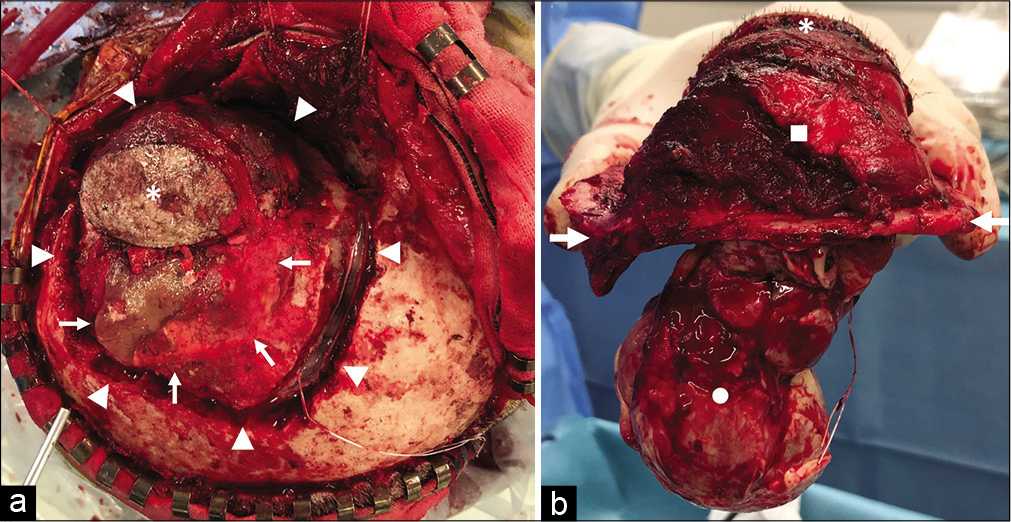
Figure 2:
Operative photographs. (a) Photograph showing that the skin and the temporal muscle covering the subcutaneous mass were left on the tumor side (white asterisk), and the skin flap was flipped. An artificial bone constructed in the previous surgery existed on the vertex side of the tumor (white arrow). A continuous narrow craniectomy was performed surrounding the tumor and the artificial bone (white arrowheads), and a dural incision was made using the space created by this narrow craniectomy. (b) Photograph showing the specimen obtained by en bloc resection of the tumor with adjacent skin (white asterisk), temporal muscle and extracranial lesion (white square), temporal bone (white arrows), dura mater, and intracranial lesion (white circle).
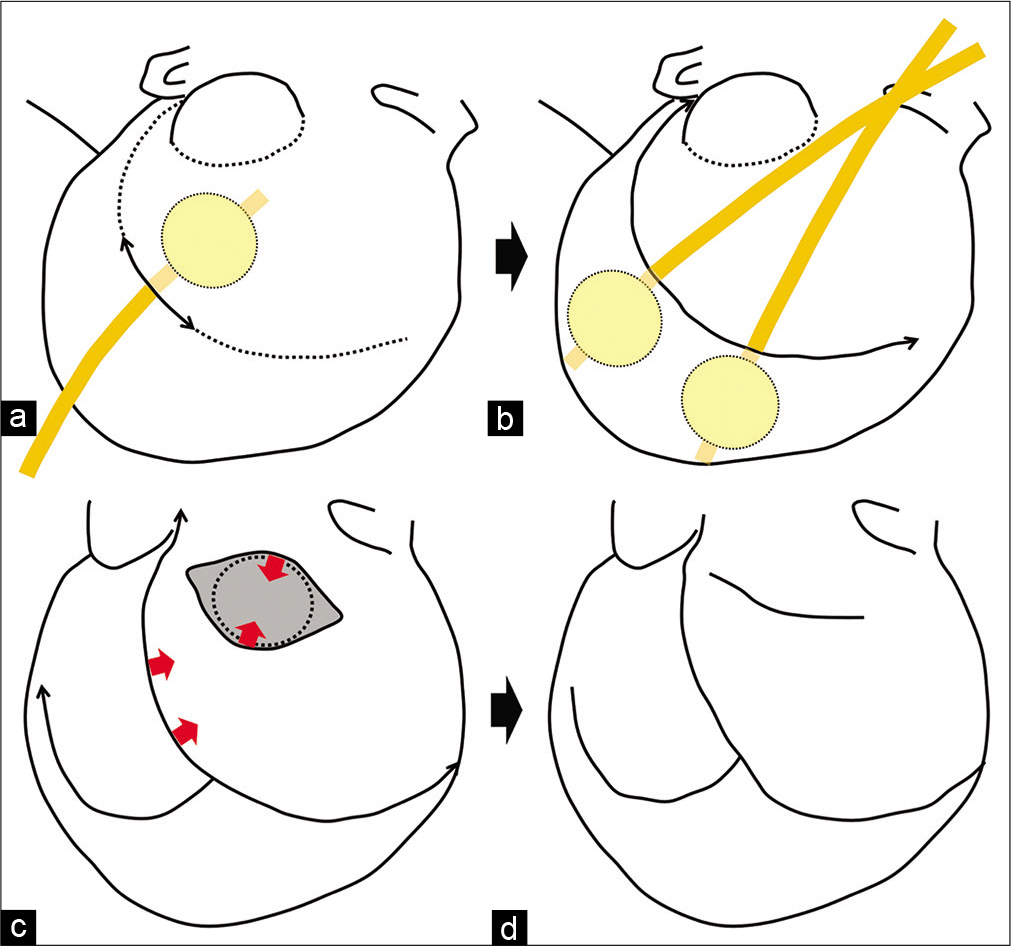
Figure 3:
Illustration of the operative procedure. (a) Illustration showing intraoperative tissue expansion of the adjacent scalp using a 30 ml balloon in a 20-French Foley catheter after a small skin incision. The Foley catheter was inserted into the subcutaneous pocket, and the balloon was inflated with 30 ml of saline for 5 min and then deflated for 3 min. (b) Illustration showing intraoperative tissue expansion of the scalp on the parietal side with two Foley catheters. Repeated expansions were performed until sufficient expansion for a primary closure was achieved. (c) Illustration showing that the local rotation skin flap was created on the dorsal side of the skin defect. (d) Illustration showing that the skin defect was covered with this rotation skin flap and the expanded surrounding scalp, and a primary closure of the skin of the head was achieved. Thin black arrows show the skin incisions. Thick black arrows show the order of the procedures. Red arrows show the direction of the skin rotation.
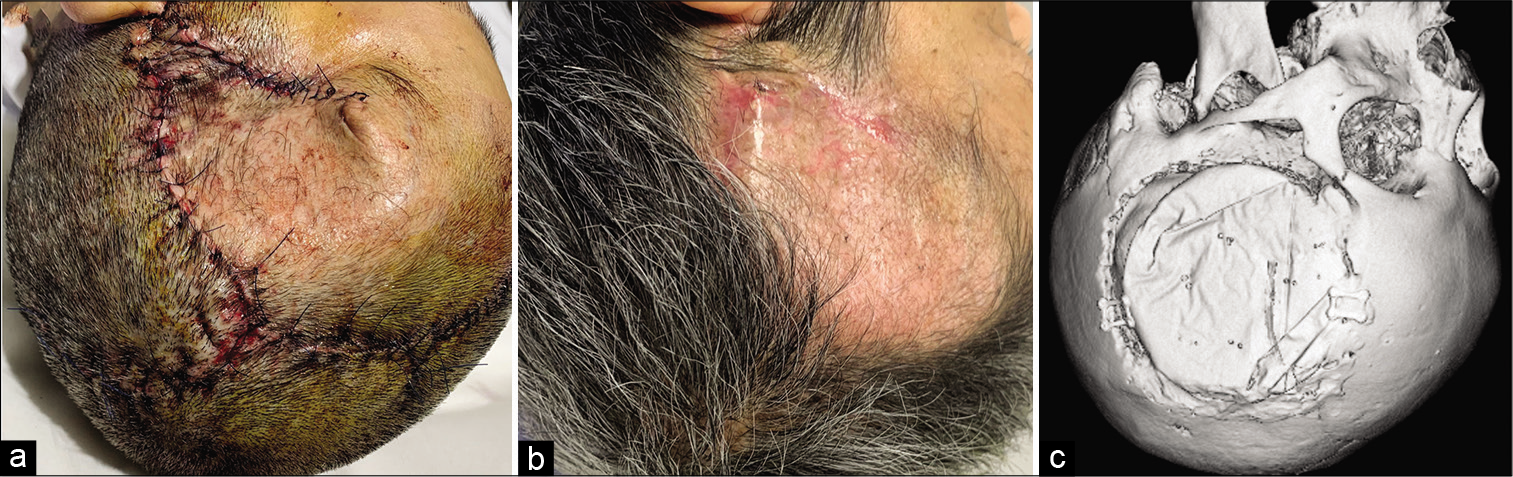
Figure 4:
Postoperative photographs and computed tomography imaging. (a) Photograph showing the surgical head wound on postoperative day 1. (b) Photograph showing the condition of the surgical head wound on postoperative day 90. (c) Postoperative computed tomography imaging showing the temporal bone defect adequately reconstructed with artificial bone.
Postoperative course
The temporal bone defect was shown to be adequately reconstructed with artificial bone on postoperative CT imaging [
DISCUSSION
In this report, we have described a case of a patient who presented with OS arising from the temporal bone 40 years after an initial surgery and radiation therapy. The nature of the OS in this patient is still unknown, though there are two possibilities. First, the tumor could have represented a recurrence of her original malignancy from residual OS cells in the temporal bone. Second, it could have been a newly developed tumor induced by the patient’s former radiation exposure. Unfortunately, information about this patient’s previous clinical course was limited. While she remembered undergoing a prior surgery in the temporal region, as well as radiation therapy, she could not remember the diagnosis of her former malignancy. Although we contacted the previous hospital to confirm this patient’s original diagnosis and her prior treatment course, her medical record was not available, and her primary surgeon was deceased. At present, we believe that the possibility of a radiation-induced secondary tumor is more likely because the 40-year latency period is likely too long for regrowth of residual tumor cells in the temporal bone.
Potential risk factors for the development of OS have been identified, including exposure to ionizing radiation.[
A comprehensive treatment strategy, including surgery, chemotherapy, and radiotherapy, is generally preferred for patients with OS. In the literature, a high mortality in head-and-neck OS is associated with difficulty in local disease control, though it demonstrates less metastasis to the lung or other sites.[
Intraoperative tissue expansion was first reported as “intraoperative sustained limited expansion” by Sasaki in 1987.[
With current multimodal treatment strategies, approximately three-quarters of all patients with OS are cured, and 90%–95% of patients with OS can be successfully treated.[
CONCLUSION
We have presented a rare case of an OS occurring from the temporal bone 40 years after radiation therapy. Our experience and the surgical methods in this case will provide options for surgical strategies in patients with head and neck OS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Takato Morioka (Department of Neurosurgery, Harasanshin Hospital) for review and supervision. We thank Prof. Yoshinao Oda, Associate Prof. Kenichi Kohashi, and Dr. Shin Ishihara (Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University) for consultation in pathological diagnosis.
References
1. Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M. NCCN guidelines insights: Bone cancer, version 2. 2017. J Natl Compr Canc Netw. 2017. 15: 155-67
2. Chen YM, Shen Q, Gokavarapu S, Lin C, Yahiya Cao W. Osteosarcoma of head and neck: A retrospective study on prognostic factors from a single institute database. Oral Oncol. 2016. 58: 1-7
3. Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer. 1983. 51: 2311-6
4. Funakoshi Y, Shono T, Kurogi A, Maehara N, Hata N, Mizoguchi M. Intraoperative tissue expansion using a Foley catheter for a scalp defect: Technical note. World Neurosurg. 2020. 143: 62-7
5. Gadwal SR, Gannon FH, Fanburg-Smith JC, Becoskie EM, Thompson LD. Primary osteosarcoma of the head and neck in pediatric patients: A clinicopathologic study of 22 cases with a review of the literature. Cancer. 2001. 91: 598-60
6. Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM. Osteosarcoma of the jaw/craniofacial region: Outcomes after multimodality treatment. Cancer. 2009. 115: 3262-70
7. Guo Z, Hu K, Zhao B, Bian E, Ni S, Wan J. Osteosarcoma of the skull base: An analysis of 19 cases and literature review. J Clin Neurosci. 2017. 44: 133-42
8. Gutiérrez VF, Pendás JL, Pelaz AC, Farpón RC, Nieto CS. Radiation-induced sarcomas of the head and neck. J Craniofac Surg. 2008. 19: 1287-91
9. Kassir RR, Rassekh CH, Kinsella JB, Segas J, Carrau RL, Hokanson JA. Osteosarcoma of the head and neck: Meta-analysis of nonrandomized studies. Laryngoscope. 1997. 107: 56-61
10. Laskar S, Basu A, Muckaden MA, D’Cruz A, Pai S, Jambhekar N. Osteosarcoma of the head and neck region: Lessons learned from a single-institution experience of 50 patients. Head Neck. 2008. 30: 1020-6
11. Lee JW, Park SH, Lee SJ, Kim SH, Jeong HS, Suh IS. New economical and simple device for intraoperative expansion on small and medium sized soft tissue defects. Arch Craniofac Surg. 2018. 19: 235-9
12. Mendenhall WM, Fernandes R, Werning JW, Vaysberg M, Malyapa RS, Mendenhall NP. Head and neck osteosarcoma. Am J Otolaryngol. 2011. 32: 597-600
13. Nishio S, Morioka T, Inamura T, Takeshita I, Fukui M, Sasaki M. Radiation-induced brain tumours: Potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien). 1998. 140: 763-70
14. Raza SM, Habib A, Wang WL, Gildey PW, Conley AP, Nader ME. Surgical management of primary skull base osteosarcomas: Impact of margin status and patterns of relapse. Neurosurgery. 2020. 86: E23-32
15. Sale KA, Wallace DI, Girod DA, Tsue TT. Radiation-induced malignancy of the head and neck. Otolaryngol Head Neck Surg. 2004. 131: 643-5
16. Sasaki GH. Intraoperative sustained limited expansion (ISLE) as an immediate reconstructive technique. Clin Plast Surg. 1987. 14: 563-73
17. Takahama A, De Abreu Alves F, Pinto CA, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 2003. 39: 521-30
18. Thariat J, Italiano A, Collin F, Iannessi A, Marcy PY, Lacout A. Not all sarcomas developed in irradiated tissue are necessarily radiation-induced-spectrum of disease and treatment characteristics. Crit Rev Oncol Hematol. 2012. 83: 393-406
19. Wood J, ver Halen J, Samant S, Florendo N. Radiation-induced sarcoma masquerading as osteoradionecrosis: Case report and literature review. J Laryngol Otol. 2015. 129: 279-82