- Serviço de Radioterapia do Hospital São Joaquim – Beneficência Portuguesa de São Paulo and Universidade Federal de São Paulo/Escola Paulista de Medicina – UNIFESP/EPM, São Paulo, S.P., Brazil
- Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, São Paulo, S.P., Brazil
- Hospital Israelita Albert Einstein, and Faculdade de Medicina da Universidade de São Paulo, São Paulo, S.P., Brazil
- Serviço de Radioterapia e Radiocirurgia do Hospital Bandeirantes, São Paulo, S.P., Brazil
Correspondence Address:
Paulo L. Moraes
Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM, São Paulo, S.P., Brazil
DOI:10.4103/2152-7806.158205
Copyright: © 2015 Moraes PL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Moraes PL, Dias RS, Weltman E, Giordani AJ, Benabou S, C. Segreto HR, Segreto RA. Outcome of cerebral arteriovenous malformations after linear accelerator reirradiation. Surg Neurol Int 04-Jun-2015;6:96
How to cite this URL: Moraes PL, Dias RS, Weltman E, Giordani AJ, Benabou S, C. Segreto HR, Segreto RA. Outcome of cerebral arteriovenous malformations after linear accelerator reirradiation. Surg Neurol Int 04-Jun-2015;6:96. Available from: http://surgicalneurologyint.com/surgicalint_articles/case-angiographically-occult-distal-small-anterior-inferior/
Abstract
Background:The aim of this study was to evaluate the clinical outcome of patients undergoing single-dose reirradiation using the Linear Accelerator (LINAC) for brain arteriovenous malformations (AVM).
Methods:A retrospective study of 37 patients with brain AVM undergoing LINAC reirradiation between April 2003 and November 2011 was carried out. Patient characteristics, for example, gender, age, use of medications, and comorbidities; disease characteristics, for example, Spetzler-Martin grading system, location, volume, modified Pollock-Flickinger score; and treatment characteristics, for example, embolization, prescription dose, radiation dose-volume curves, and conformity index were analyzed. During the follow-up period, imaging studies were performed to evaluate changes after treatment and AVM cure. Complications, such as edema, rupture of the blood-brain barrier, and radionecrosis were classified as symptomatic and asymptomatic.
Results:Twenty-seven patients underwent angiogram after reirradiation and the percentage of angiographic occlusion was 55.5%. In three patients without obliteration, AVM shrinkage made it possible to perform surgical resection with a 2/3 cure rate. A reduction in AVM nidus volume greater than 50% after the first procedure was shown to be the most important predictor of obliteration. Another factor associated with AVM cure was a prescription dose higher than 15.5 Gy in the first radiosurgery. Two patients had permanent neurologic deficits. Factors correlated with complications were the prescription dose and maximum dose in the first procedure.
Conclusion:This study suggests that single-dose reirradiation is safe and feasible in partially occluded AVM. Reirradiation may not benefit candidates whose prescribed dose was lower than 15.5 Gy in the first procedure and initial AVM nidus volume did not decrease by more than 50% before reirradiation.
Keywords: Arteriovenous malformations, radiosurgery, reirradiation
INTRODUCTION
Arteriovenous malformation (AVM) of the brain is a vascular alteration of embryonal origin, with a network of tangled vessels also named nidus. Although benign, AVM may have major clinical repercussions due to the possibility of rupture.[
Radiosurgery is an important treatment modality for both malignant and benign intracranial disorders.[
Reirradiation performed with Gamma Knife shows that around 60–70% of patients with partially obliterated AVM after the first radiosurgery achieve occlusion.[
Despite good AVM obliteration rates with radiosurgery, adverse effects such as edema, rupture of the blood–brain barrier, brain cyst formation, and brain radionecrosis may occur.[
Factors attributed to the patient, disease, and treatment contributes either to AVM obliteration or an increased incidence of complications after brain radiosurgery.[
Thus, the purpose of this study was to assess the predictors of response and complications in AVM patients undergoing reirradiation with LINAC radiosurgery.
MATERIALS AND METHODS
A retrospective study including patients with brain AVM, undergoing reirradiation with radiosurgery, was conducted from April 2003 to November 2011, in the São Joaquim Hospital – Beneficência Portuguesa de São Paulo in cooperation with São Paulo Federal University, Radiotherapy Department.
In 156 patients diagnosed with AVM, 41 (26.2%) were reirradiated after undergoing follow-up brain angiography with the presence of residual AVM nidus. Of these patients, 37 (37/41: 90%) met the inclusion criteria of the study: Persistence of nidus 3 years after radiosurgery; age over 18 years; access to treatment plan; clinical and imaging follow-up longer than 6 months.
Data were retrospectively collected and included patient variables (gender, age, use of medications), disease (location, volume), and treatment (embolization, prescription dose, radiotherapy dose–volume curves of 6, 8, 10, 12, 14, 16, 18, 20 Gy, dose–volume curves in the normal tissue, radiotherapy dose in the following volumes: 10, 16, 20, 24 cm3 including and excluding nidus, and conformity index). The modified Pollock–Flickinger score scale was used in 27 patients undergoing angiography after reirradiation.
Treatment planning included magnetic resonance imaging (MRI) of the brain (1.3 mm slices) and also computed tomography (CT) (0.6 mm slices) in the supine position. During CT scan, the patient was immobilized with a stereotactic arc (frame), or thermoplastic mask (Brain Lab AG, Feldkirchen, Germany). Treatment planning angiography was then performed, with the acquisition of two films, one lateral and one anterior view.
Images obtained were sent to the Brain Scan Planning System, version 5.3 or Iplan version 4.1. Fusion of CT image was performed with MRI and angiography images for structure location. The nidus and critical structures were outlined.
Dose distribution, prescription dose and tolerance dose of organs at risk were also defined. The dose was normalized to 100% and prescription in the 80% curve encompassing the entire nidus.
Treatment was performed in LINAC 600C and Novalis (Varian/Brain Lab, Palo Alto, USA). Head frame immobilization was used in patients treated in the LINAC 600C, and a map was printed and fixed to the target positioner box for isocenter location. When treatment was performed in the Novalis LINAC, the BrainLab ExacTrac system was used to locate the isocenter (Varian/Brain Lab, Palo Alto, USA).
Treatment was delivered with a micromultileaf collimator (3 mm) (Varian/Brain Lab, Palo Alto, USA) or cones (Integra Radionics, Burlington, USA).
Routine follow-up care of the patient consisted of clinical examination and brain MRI every 4 months in the first 2 years, every 6 months from the third to fifth year and annually after this period. At 3 years of follow-up, patients underwent angiography after reirradiation (27/37: 72.3%).
Statistical analysis
A statistical descriptive analysis was performed for categorical variables related to patient, lesion and treatment characteristics (primary and reirradiation) and results were expressed in percentages.
For the association between angiographic cure and categorical variables, the Fisher exact test was used. For numerical variables (dose, nidus volume, number of nutrition vessels, conformity index, number of fields, patient age), the Mann–Whitney test was used. For multivariate analysis of significant data related to cure or complications, a logistic regression analysis model with stepwise variable selection was used.
Cut-off values for numerical variables and their associations were established using the Receiver Operating Characteristics (ROC) curve. The level of significance adopted was P ≤ 0.05.
Statistical analyses were performed with the Statistical Package software for the Social Sciences (SPSS), version 15.0 for Windows and R-Program, version 2.9.2.
RESULTS
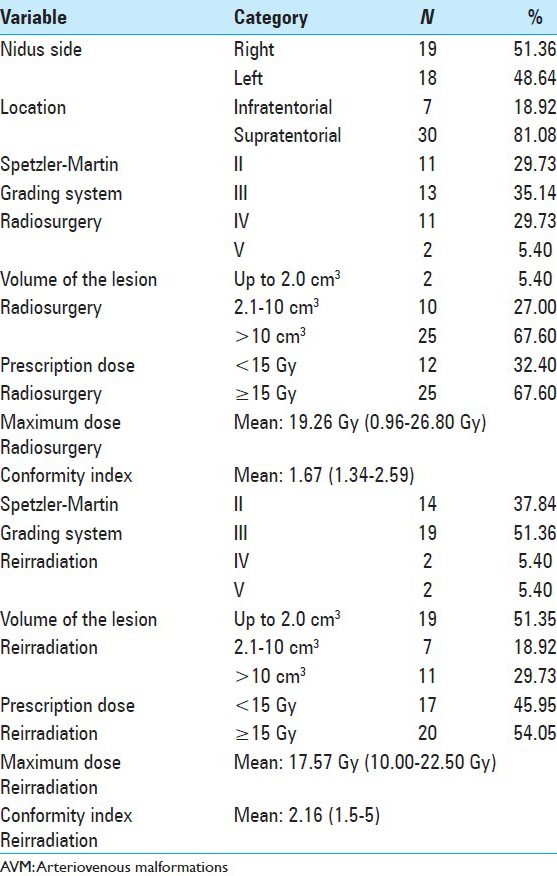
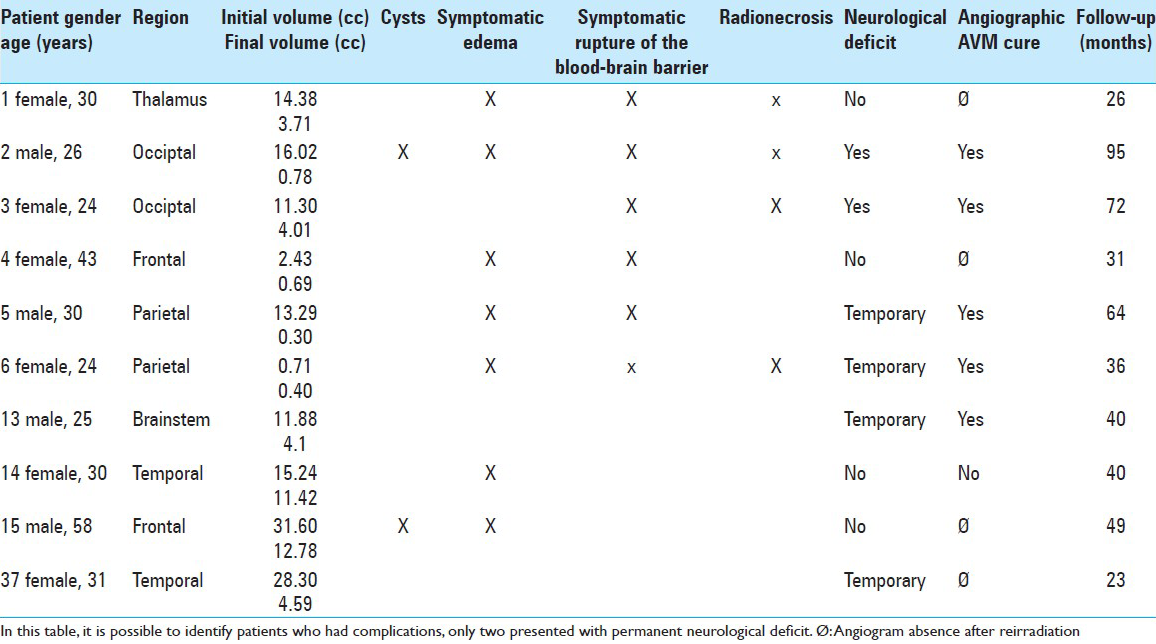
The results relative to patient characteristics showed a predominance of Caucasians, female gender, age ranging from 18 to 25 years old, and use of anticonvulsants by half of the patients. Concerning treatment, the majority of patients were classified as high-grade Spetzler–Martin (SMG), with a nidus volume larger than 10 cm3 (67.6%) in the first radiosurgery. Other factors concerning the patients and treatment can be seen in [
AVM were distributed among seven different regions. No significant correlation between nidus location and symptomatic complication was found in our study.
In the first radiosurgery, initial nidus volume ranged from 0.61 to 52.5 cm3, with a mean value of 16.6 cm3. The mean prescribed dose was 15 Gy, ranging from 8 to 20 Gy. The mean time period for reirradiation was 42 months (36.5–71.5 months).
The mean nidus volume at reirradiation was 6.5 cm3, ranging from 0.07 to 34 cm3. The mean prescribed dose in the second procedure was 14.1 Gy (10–18 Gy).
The modified Pollock–Flickinger (mPF) scale before reirradiation showed that the percentage of patients with AVM obliteration and no new permanent deficits was 54.54% for patients who scored <1.00 (6/11, eight patients had angiographic cure but two presented a new neurologic deficit); 77.77% (7/9) for patients who scored 1.01–1.50; and 0% for patients who scored higher than 1.51 (0/7).
When values were grouped using a cut-off point of 1.5 in the mPF scale, 65% of patients who scored ≤1.5 had a favorable outcome (13/20), and none of the 7 patients who scored higher than 1.5 had a favorable outcome with statistical significance for this variable in the univariate analysis (P = 0.006).
After reirradiation, the mean follow-up period was 47.6 months, ranging from 7 to 95 months. Fifteen patients showed complete AVM obliteration after angiography (15/27: 55.5%) at a mean period of 40.88 months, and 12 had residual nidus on the angiogram. Three patients with incomplete nidus obliteration underwent surgical AVM resection. Therefore, 2/3 of the patients achieved AVM cure during this period (angiographically or surgically).
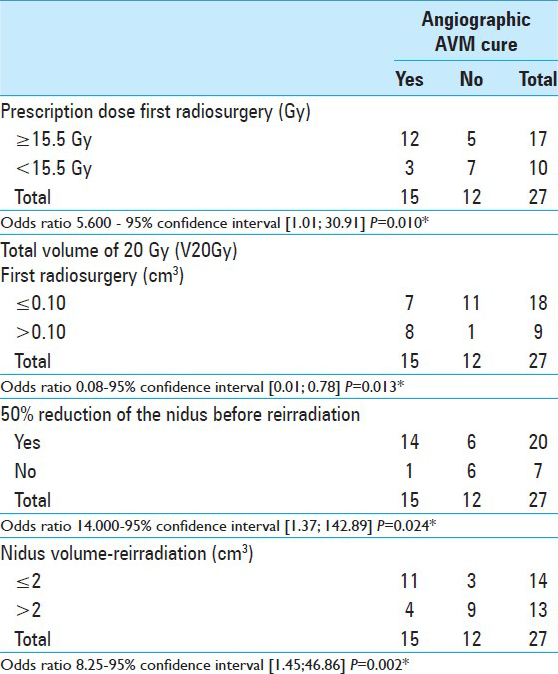
Factors related to angiographic cure were reduction in nidus volume greater than 50% before reirradiation, initial prescribed dose ≥15.5 Gy, volumes of total and normal tissue receiving 20 Gy in the first procedure and nidus volume ≤2 cm3 before reirradiation [Tables
Factors related to asymptomatic and symptomatic edema was the maximum dose in the first procedure ≥18.94 Gy. Factors related to rupture of the blood-barrier were prescribed dose ≥15.5 Gy in the first procedure and reirradiation nidus volume ≤1.5 cm3 [
During the first radiosurgery, the mean volume of tissue receiving a dose of 12 Gy (V12) excluding the AVM target volume from the considered irradiated volume (Vn12) was 35.3 cm3 and 20.6 cm3, respectively. For those who had symptomatic complications, V12 was 34.3 cm3 and Vn12 was 20.03 cm3. In patients who did not have complications, V12 was 35.7 cm3 and Vn12 was 20.86 cm3. No significant correlation between symptomatic complications and dose volumes in the first radiosurgery was found.
At reirradiation, the mean V12 and Vn12 was 15.9 and 9 cm3, respectively. For patients with symptomatic complications V12 was 11.8 cm3 and Vn12 was 7.6 cm3. In those who had no complications, V12 was 17.5 cm3 and Vn12 was 9.46 cm3. No significant correlation was found between symptomatic complications and dose–volume effects at reirradiation.
During the first radiosurgery, the mean dose for a volume of 20 cm3 (Dm20) and excluding the AVM target volume from the considered irradiated volume (Dn20) was 13 and 8.08 Gy. For those with symptomatic complications, Dm20 was 12.7 Gy and Dn20 was 7.95 Gy. In those who had no complications, Dm20 and Dn20 was 13.08 and 8.12 Gy. No significant correlation between symptomatic complications and mean doses at specific volumes was found during the first radiosurgery.
The Dm20 and Dn20 was 9.16 and 6.9 Gy at reirradiation. No significant correlation between symptomatic complications and mean doses at specific volumes was found at reirradiation.
Six patients were symptomatic for edema after reirradiation and symptoms secondary to edema occurred at a mean time of 15.47 months (range: 3–49 months). Rupture of the blood–brain barrier occurred in 10 patients after reirradiation, ranging from 1 to 30 months, and five patients had symptoms, at a mean time of 17.5 months (7–22 months).
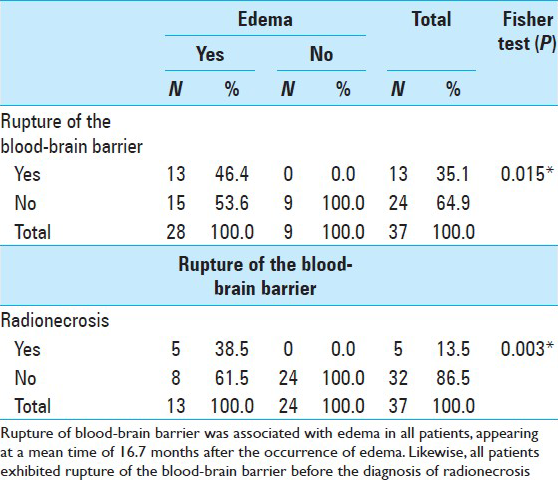
Barrier rupture was associated with edema in all patients, appearing at a mean time of 16.7 months (0–45 months) after the occurrence of edema. Five patients had radionecrosis detected by imaging tests. Of these, three were identified after reirradiation. The mean time for the appearance of radionecrosis was 29 months, with an interval of 18.8–45.9 months.
All patients exhibited rupture of the blood–brain barrier before the diagnosis of radionecrosis. There was a significant correlation between both complications [
Ten patients (27%) had symptomatic complications. Two of those patients (5.4%) presented with permanent neurologic deficit and four had transitory deficits. Two patients had cysts adjacent to the AVM. Two episodes of bleeding occurred after reirradiation, at 7 and 54 months [
DISCUSSION
Data on patient characteristics showed that mean nidus volume decreased significantly from 16 to 6.5 cm3. Patients with SMG IV and V decreased from 35.1% in the first radiosurgery to 10.8% at reirradiation. In the majority of cases, there was a decrease in AVM volume and migration to a group classified as SMG grade II and III, in agreement with the literature.[
In our study, the angiographic cure rate was 55.5%, at a mean time period of 40.8 months. These values were similar to findings for LINAC treatment.[
Yamamoto et al., assessed AVMs larger than 10 cm3 in 26 patients who received relatively low doses of radiotherapy (12–16 Gy) and reirradiation after 3 years. Twenty patients received follow-up brain angiography showing 65% of nidus obliteration.[
Repeat radiosurgery was used before, as shown by Karlson et al. Those authors found a 62% occlusion rate in 133 patients with a nidus volume of 9 ml or more.[
In a study by Kano et al.,[
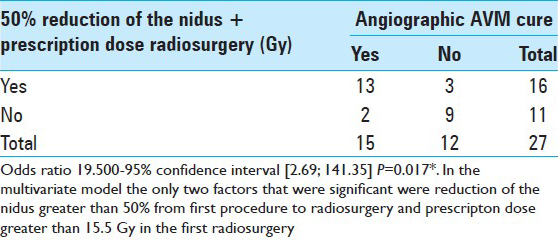
The cut-off value for the prescribed dose associated with AVM occlusion in the first radiosurgery was established using the ROC curve. A prescribed dose ≥15.5 Gy in the first procedure had an odds ratio of 5.6 for AVM obliteration, in comparison to lower doses. With the addition of both factors, reduction in nidus size greater than 50% and prescribed dose ≥15.5 Gy in the first radiosurgery, the odds ratio for occlusion was 19.5 times higher than in patients without these factors. In multivariate analysis, these were the only two significant factors for nidus occlusion before reirradiation.
Volume was also a predictor of nidus occlusion at reirradiation. In our study, the cut-off point for nidus volume was ≤2.0 cm3 at reirradiation. In the largest study of patients reirradiated using LINAC, nidus volumes during the first radiosurgery and reirradiation were similar to those of our study. Stahl et al., observed that cure was related to a smaller nidus volume, a high prescription dose and male gender.[
Other factors such as age, gender, previous embolization, conformity index, dose–volume curves, did not correlate with AVM obliteration. In contrast, findings published in a recent study correlated some of these factors with a higher obliteration rate.[
In our study, 5.4% of the patients had permanent neurologic deficits and complications were similar to those found in the literature. In the majority of studies, the frequency of neurologic deficits ranged from 3% to 10%.[
The only factor that correlated significantly with edema was the maximum dose in the first procedure ≥ 18.94 Gy. Factors related to rupture of the blood barrier were prescription dose in the first procedure ≥15.5 Gy and nidus volume before reirradiation ≤1.5 cm3. The latter was probably related to the delivery of higher doses to small AVM nidus. According to Stahl et al., a factor that increased the complication rate was also the prescription dose, in addition to AVM location.[
In our study, AVM were located in seven areas. There was no significant correlation between nidus location and any type of complication.
Flickinger et al., showed that nidus location dramatically affected whether or not changes in postradiosurgery imaging were symptomatic. However, none of the individual locations had a significant effect on complications and a significant postradiosurgery imaging expression (SPIE) score was constructed.[
According to the SPIE score, the frontal and temporal areas are low-risk regions that represented 43.2% of our patients. Ten patients had symptomatic complications as follows: 2 frontal, 2 temporal, 2 parietal, 0 cerebellar, 2 occipital, 1 thalamus, and 1 brainstem.
In 2008, Pollock and Flickinger developed a simplified radiosurgery-based AVM grading system based on factors associated with a successful outcome. Angiographic obliteration without new deficits was predicted using a two-tiered ranking of location (basal ganglia/thalamus/brainstem versus other).[
The modified Pollock–Flickinger scale was used in patients undergoing angiography after reirradiation. Perhaps statistical significance was not found for this variable due to the limited number of patients. Although patients were divided into two groups and 65% who scored ≤1.5 had a favorable outcome, none of these patients had nidus occlusion when scores were higher than 1.5 and this was statistically significant (P = 0.006).
The largest study of LINAC AVM retreatment[
Levegrun et al., showed that a mean dose 18.9 Gy to a volume of 20 cm3 (Dm20) and a volume of 27.6 cm3 receiving a dose of 12 Gy (V12) had a 2.8-fold higher risk of developing edema and/or blood–brain barrier rupture than those with V12 of 4.2 cm3 and Dm20 of 8.4 Gy. There was a low degree of accuracy in predicting complications when the AVM target volume was excluded from the considered irradiated volume.[
Seventy-five percent of the patients presented edema visualized by imaging tests. Of these, 18 had edema after the first procedure and 10 after reirradiation. In a study of 85 patients, Hayhurst et al., observed that 50% of those patients exhibited some alteration on serial T2-weighted MRI.[
Rupture of the blood–brain barrier has been associated with previous edema in all patients, corroborating observations by Buis et al. Those authors described that hyperintensity surrounding the nidus before reirradiation was predictive of an increased incidence of side-effects, affecting 20% of patients.[
Thirteen patients had rupture of the blood–brain barrier. Of these, five progressed to radionecrosis. A statistically significant correlation between both disorders has been observed. The pathophysiology of radionecrosis may explain this finding, since it is well-known that glial and endothelial cellular lesion generated by radiosurgery causes rupture of the blood–brain barrier, which may be accompanied by radionecrosis.[
Three patients had radionecrosis after reirradiation, with a mean time of 29 months. Schlienger et al., demonstrated similar results in their study, describing that 9% of the patients had neurologic deficits and radionecrosis did not increase significantly.[
In our study, the incidence of cysts was low and occurred in two symptomatic patients who were treated surgically. Two patients (5.4%) presented with bleeding resulting from the AVM. Studies in patients undergoing repeat irradiation have shown that bleeding rates range from 0% to 9%.[
CONCLUSION
Taken together, our results showed that reirradiation with LINAC radiosurgery was a feasible procedure for salvage of AVM patients, who did not obtain AVM obliteration after first irradiation. Reirradiation may not benefit candidates whose prescribed dose was lower than 15.5 Gy in the first procedure and initial nidus volume did not decrease by more than 50% before reirradiation.
References
1. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010. 77: 996-1001
2. Buis DR, Meijer OW, van den Berg R, Lagerwaard FJ, Bot JC, Slotman BJ. Clinical outcome after repeated radiosurgery for brain arteriovenous malformations. Radiother Oncol. 2010. 95: 250-6
3. Colombo F, Benedetti A, Pozza F, Zanardo A, Avanzo RC, Chierego G. Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol. 1985. 48: 133-45
4. D’Ambrosio AL, DeYoung C, Isaacson SR. Radiosurgical management of brain metastases. Neurosurg Clin N Am. 2011. 22: 45-51
5. Flickinger JC. An integrated logistic formula for prediction of complications from radiosurgery. Int J Radiat Oncol Biol Phys. 1989. 17: 879-85
6. Flickinger JC, Schell MC, Larson DA. Estimation of complications for linear accelerator radiosurgery with the integrated logistic formula. Int J Radiat Oncol Biol Phys. 1990. 19: 143-8
7. Flickinger JC, Lunsford LD, Kondziolka D, Maitz AH, Epstein AH, Simons SR. Radiosurgery and brain tolerance: An analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 1992. 23: 19-26
8. Flickinger JC, Kondziolka D, Pollock BE, Maitz AH, Lunsford LD. Complications from arteriovenous malformation radiosurgery: Multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys. 1997. 38: 485-90
9. Flickinger JC, Kondziolka D, Lunsford LD, Kassam A, Phuong LK, Liscak R. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000. 46: 1143-8
10. Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002. 63: 347-54
11. Friedman WA, Foote KD. Linear accelerator radiosurgery in the management of brain tumors. Ann Med. 2000. 32: 64-80
12. Hayhurst C, Monsalves E, Van Prooijen M, Cusimano M, Tsao M, Menard C. Pretreatment predictors of adverse radiation effects after radiosurgery for arteriovenous malformation. Int J Radiat Oncol Biol Phys. 2012. 82: 803-8
13. Kano H, Kondziolka D, Flickinger JC, Yang H, Flannery TJ, Awan NR. Stereotactic Radiosurgery for Arteriovenous Malformations, Part 3: Outcome predictors and risks after repeat radiosurgery. J Neurosurg. 2012. 116: 21-32
14. Karlsson B, Jokura H, Yamamoto M, Soderman M, Lax I. Is Repeated radiosurgery an alternative to staged radiosurgery for very large brain arteriovenous malformations?. J Neurosurg. 2007. 107: 740-4
15. Karlsson B, Kihlström L, Lindquist C, Steiner L. Gamma knife surgery for previously irradiated arteriovenous malformations. Neurosurgery. 1998. 42: 1-5
16. Knoos T, Kristensen I, Nilsson P. Volumetric and dosimetric evalution of radiation treatment plans: Radiation conformity index. Int J Radiat Oncol Biol Phys. 1998. 42: 1169-76
17. Leksell L. Cerebral radiosurgery. I Gammathalanotomy in two cases of tractable pain. Acta Chir Scand. 1968. 134: 585-95
18. Levegrun S, Hof H, Essig M, Schlegel W, Debus J. Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: Correlation with dose distribution parameters. Int J Radiat Oncol Biol Phys. 2009. 59: 796-808
19. Maesawa S, Flickinger JC, Kondziolka D, Lunsford LD. Repeated radiosurgery for incompletely obliterated arteriovenous malformations. J Neurosurg. 2000. 92: 961-70
20. Marks LB, Spencer DP. The influence of volume on the tolerance of the brain to radiosurgery. J Neurosurg. 1991. 75: 177-80
21. Mirza-Aghazadeh J, Andrade-Souza YM, Zadeh G, Scora D, Tsao MN, Schwartz ML. Radiosurgical retreatment for brain arteriovenous malformation. Can J Neurol Sci. 2006. 33: 189-94
22. Nedzi LA, Kooy H, Alexander E, Gelman RS, Loeffler JS. Variables associated with the development of complications from radiosurgery of intracranial tumors. Int J Radiat Oncol Biol Phys. 1991. 21: 591-9
23. Nieder C, Andratschke N, Astner ST. Experimental concepts for toxicity prevention and tissue restoration after central nervous system irradiation. Radiat Oncol. 2007. 2: 23-
24. Pollock BE, Flickinger JC. Modification of the Radiosurgery-based Arteriovenous Malformation Grading System. Neurosurgery. 2008. 63: 239-43
25. Schlienger M, Nataf F, Lefkopoulos D, Mammar H, Missir O, Meder JF. Repeat Linear Accelerator Radiosurgery for Cerebral Arteriovenous Malformations. Int J Radiat Oncol Biol Phys. 2003. 56: 529-36
26. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. Neurosurgery. 1986. 65: 476-83
27. Stahl JM, Chi Y, Friedman WA. Repeat Radiosurgery for Intracranial Arteriovenous Malformations. Neurosurgery. 2012. 70: 150-4
28. Steiner L, Leksell L, Greitz T, Forster DM, Backlund EO. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972. 138: 459-64
29. van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJ. Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA. 2011. 306: 2011-9
30. Yamamoto M, Akabane A, Matsumaru Y, Higuchi Y, Kasuya H, Urakawa Y. Long-term follow-up results of intentional 2-stage Gamma Knife surgery with an interval of at least 3 years for arteriovenous malformations larger than 10 cm3. J Neurosurg. 2012. 117: 126-34
31. Yen CP, Jain S, Haq IU, Jagannathan J, Schlesinger D, Sheehan J. Repeat γ knife surgery for incompletely obliterated cerebral arteriovenous malformations. Neurosurgery. 2010. 67: 55-64
32. Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008. 25: 51-8
33. Zabel A, Milker-Zabel S, Huber P, Schulz-Ertner D, Schlegel W, Debus J. Treatment outcome after Linac-based radiosurgery in cerebral arteriovenous malformations: Retrospective analysis of factors affecting obliteration. Radiother Oncol. 2005. 77: 105-10