- Departments of Neurology, Riverside Community Hospital, CA, United States.
- Departments of Medicine, Riverside Community Hospital, CA, United States.
- Department of Medicine, University of California, CA, United States.
- Department of Surgery, Riverside Community Hospital, Riverside, CA, United States.
Correspondence Address:
Sara Zarei
Department of Surgery, Riverside Community Hospital, Riverside, CA, United States.
DOI:10.25259/SNI_60_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sara Zarei1, Alina Popa2, Bahman Moghadam2, Archana Reddy3, Ahmed Mahmoud4. Oversized primary intrapulmonary schwannoma: A case report and a review of the literature. 08-Aug-2020;11:234
How to cite this URL: Sara Zarei1, Alina Popa2, Bahman Moghadam2, Archana Reddy3, Ahmed Mahmoud4. Oversized primary intrapulmonary schwannoma: A case report and a review of the literature. 08-Aug-2020;11:234. Available from: https://surgicalneurologyint.com/surgicalint-articles/10192/
Abstract
Background: Schwannomas, also known as neurilemommas, are benign, well-circumscribed encapsulated peripheral nerve sheath tumors with rather indolent evolution. Made up of cells closely related to normal myelinating Schwann cells, these neoplasms may arise from the peripheral nervous system as well as from spinal or cranial nerves. They are mostly found in the base of the skull, neck, chest wall, posterior mediastinum, posterior spinal roots, cerebellopontine angle, retroperitoneum, and flexor surfaces of the extremities. The incidence rate of spinal schwannoma is 0.3–0.5/100,000 cases per year with an average age of 50 at diagnosis. We report a case of intrapulmonary schwannoma, adding a review of the literature.
Case Description: A 20-year-old female patient with no significant medical history, presented with pleuritic chest pain, shortness of breath, right upper limb weakness, and numbness. A computed tomography of the chest and magnetic resonance imaging showed a 7.2 × 10.5 × 8.3 cm mass in the posterior segment of the right upper lobe, arising from the right T5-6 neural foramen; a concurrent 16 mm thick right pleural effusion was also noticed yet without evidence of nodular enhancement. The findings suggested the presence of a neurofibroma or a schwannoma. Complete resection of the tumor was achieved through posterolateral thoracotomy; the ensuing histopathological and immunohistochemical examinations confirmed the presence of a schwannoma.
Conclusion: We believe this rare case of pulmonary invasive schwannoma illustrates the complex dynamics of this extremely rare entity; in this particular case, complete surgical excision proved to be crucial in terms of subacute management and local tumor control, at least at short and middle term. The patient is currently asymptomatic (6 months postsurgery) and remains on follow-up.
INTRODUCTION
Schwannomas, also called neurilemmomas, are well-circumscribed encapsulated peripheral nerve sheath tumors. Histopathologically, these neoplasms are made up of cells closely related to the normal Schwann cells that myelinate the peripheral nervous system.[
The incidence rate of spinal schwannoma is 0.3–0.5/100,000 cases per year and the average age at diagnosis is around 50.[
The pattern of growth of schwannomas is usually slow and often indolent from a clinical perspective; as such, only selected cases undergo surgery.[
We report a case of a large intrapulmonary schwannoma, adding a review of the current literature.
CASE DESCRIPTION
A previously healthy 20-year-old female patient presented at the emergency department with 4 days history of concurrent severe right-sided pleuritic chest pain and moderate ipsilateral clavicular pain, shortness of breath on exertion, as well as combined right upper limb weakness and bilateral fingertip numbness. No other symptoms were reported. She accounted no recent travel or family history, including neurofibromatosis.
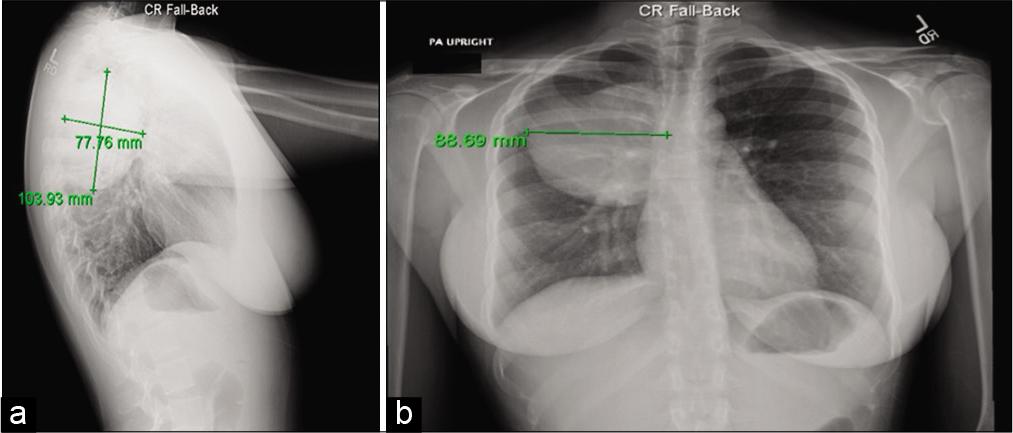
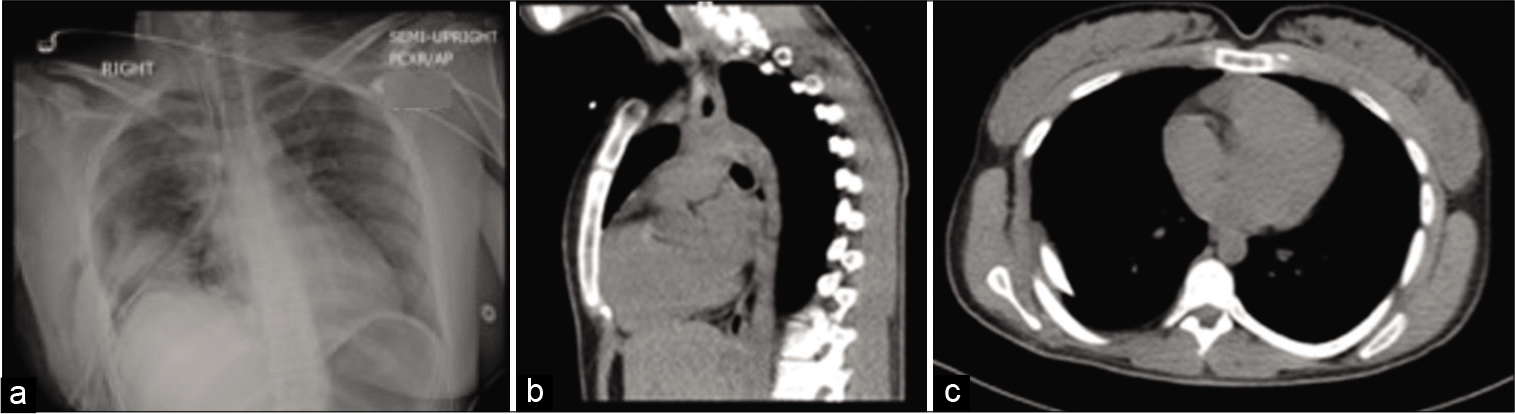
On admission, the ECG and troponins were found to be within normal limits. However, the ensuing chest X-ray (CXR) showed a large oval mass-like density in the posterior segment of the right upper lobe [
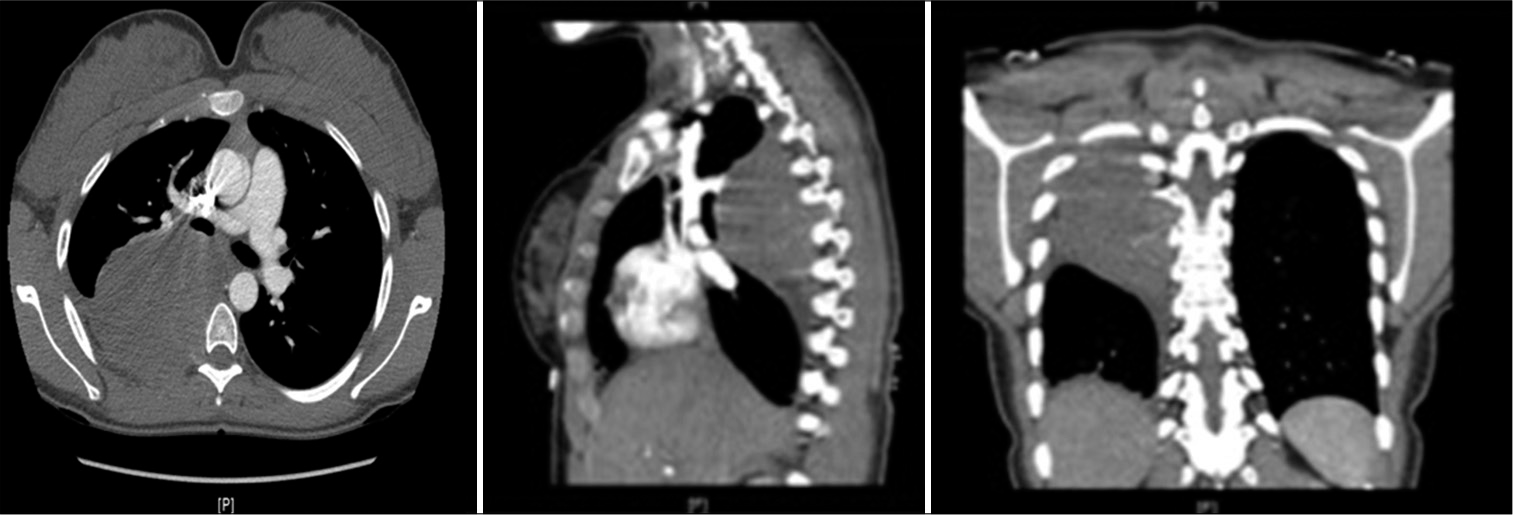
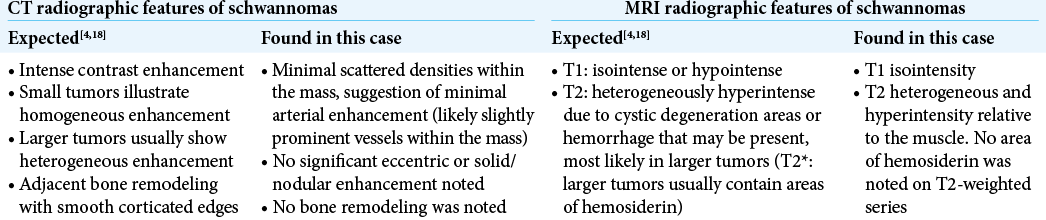
The complementary CT scan of the chest with contrast showed a low density 7.2 × 10.5 × 8.3 cm mass in the posterior segment of the right upper lobe with no nodular enhancement, potentially originating at the right T5-T6 neural foramen. A 16 mm thick right pleural effusion was also described [
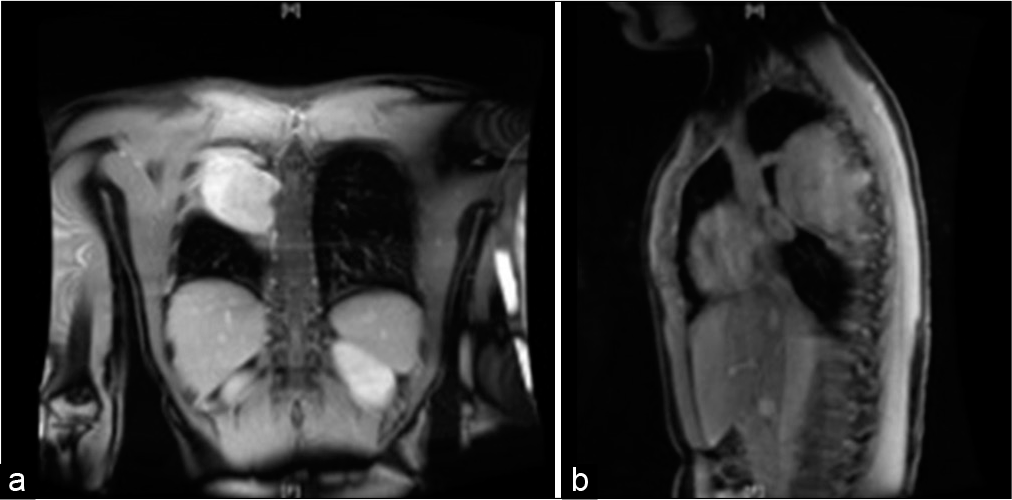
The ensuing MRI with gadolinium demonstrated a large (at least 7.2 × 9.2 × 8.5 cm) T1 isointense and a mild T2 hyperintense (heterogenic) mass relative to the muscle and within the right upper/mid posterior hemithorax. A focal contrast-enhancing protrusion of the right posterior medial aspect of the mass extending into the right T5-6 neural foramen suggested that the mass emanated from the neural foramen [
Figure 3:
(a and b) Preoperative magnetic resonance imaging gadolinium of the chest demonstrating a large, mildly heterogenic hyperenhancing mass within the posterior right upper/mid- hemithorax (7.2 × 9.2 × 8.5 cm) extending to the level of the right C5-6 neural foramen. T1 isointensity and mild T2 hyperintensity relative to muscle are reported. Small right pleural effusion, up to 13 mm is noted. No other pathological findings are observed.
In view of the patient’s age, concurrent symptomatology, Karnofsky Performance Scale (KPS) of 70 (mainly due to presenting symptoms), size of the lesion, and topoanatomical constraints, we decided to remove the tumor using a right- sided posterolateral thoracotomy followed by a perilesional dissection; the mass was successfully freed from the corresponding posterior rib and transverse process. We then proceeded to follow the pedicle into the right T5-T6 foramen, ultimately achieving “en bloc” tumor resection including the proximal nerve root. No spinal fluid leak or persistent bleeding was noted; the surgical site was closed accordingly and without complications.
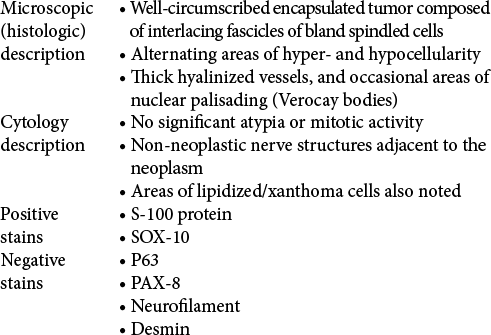
The microscopic examination of the collected samples demonstrated a well-circumscribed encapsulated tumor composed of interlacing fascicles of bland spindled cells with alternating areas of hyper- and hypocellularity, thick hyalinized vessels, and occasional areas of nuclear palisading (Verocay bodies). Areas of lipidized/xanthoma cells were also noted. Non-neoplastic nerve structures were found adjacent to the neoplasm. No significant atypia or mitotic activity was seen. Finally, the immunohistochemical staining proved positive for S-100 protein, SOX-10 while negative for p63, PAX-8, neurofilament, and desmin. The findings confirmed the diagnosis of schwannoma [
The patient was transferred to the intensive care unit postoperatively; her evolution at the hospital proved uncomplicated with follow-up serial CXRs remaining unremarkable [
Figure 4:
(a) Chest X-ray 24 h postsurgery; Right-sided chest tube and mediastinal drain are noted. No evidence of residual pneumothorax. A right internal jugular catheter is noted with the distal tip in the region of the right atrium. Interval resection of the previously noted right mediastinal mass. No evidence of pleural effusion. Osseous structures remain unremarkable. (b) and (c): CT chest a month postsurgery: normal findings, no evidence of recurrence.
DISCUSSION
A study of 75 peripheral nerve sheath tumor cases done in 2015 showed that among intrathoracic peripheral nerve sheath tumors, 75% of cases are found in the posterior mediastinum in association with paraspinal nerves and soft-tissue sites.[
The majority of peripheral nerve sheath tumors arise sporadically, but some can be seen in the context of neurofibromatosis Types 1 and 2. In addition, most of these tumors are of benign nature and can be further subclassified as schwannoma, neurofibromas, and perineuromas.[
One of the key diagnostic tools used when studying these peripheral nerve sheath tumors is immunohistochemistry; as illustrated by our case, S-100 remains a common histochemical features for neurofibromas, schwannomas, perineurioma, and ganglioneuroma.[
The imaging screening of intrathoracic peripheral nerve sheath tumors begins with a CXR; the ensuing CT scan usually describes the presence of a “well-circumscribed smooth mass.”[
Complete surgical excision is the mainstay of treatment for peripheral nerve sheath tumors as it serves a diagnostic and therapeutic purpose.[
Finally, depending on the size, location, and site of invasion of the tumor, a thoracotomy or video-assisted thoracotomy surgery (VATS) can be undertaken; the surgical indications for the aforementioned procedure can be found elsewhere.[
CONCLUSION
This case report shows how an oversized intrapulmonary schwannoma can remain asymptomatic for a long period of time; symptoms may rapidly evolve due to the compression of adjacent structures in a mass effect manner. In these cases, the medical literature advocates for complete surgical excision of the tumor which was achieved in our case. We believe the aforementioned strategy had a positive influence on local tumor control and survival; however, the latter needs to be confirmed by a 5 years follow-up.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ando K, Imagama S, Ito Z, Kobayashi K, Yagi H, Hida T. How do spinal schwannomas progress? The natural progression of spinal schwannomas on MRI. J Neurosurg Spine. 2016. 24: 155-9
2. Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: Radiologic-pathologic correlation. Radiographics. 2004. 24: 1477-81
3. Boland JM, Colby TV, Folpe AL. Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 Cases. Hum Pathol. 2015. 46: 419-25
4. Brant WE, Helms CA.editors. Fundamentals of Diagnostic Radiology. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. p.
5. Fehlings MG, Nater A, Zamorano JJ, Tetreault LA, Varga PP, Gokaslan ZL. Risk factors for recurrence of surgically treated conventional spinal schwannomas: Analysis of 169 patients from a multicenter international database. Spine (Phila Pa 1976). 2016. 41: 390-8
6. Hanna JM, Berry MF, D’Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis. 2013. 5: S182-9
7. Huang JH, Johnson VE, Zager EL. Tumors of the peripheral nerves and plexuses. Curr Treat Options Neurol. 2006. 8: 299-308
8. Jeon JH, Hwang HS, Jeong JH, Park SH, Moon JG, Kim CH. Spinal schwannoma; analysis of 40 Cases. J Korean Neurosurg Soc. 2008. 43: 135-8
9. Karamchandani JR, Nielsen TO, Van De Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012. 20: 445-50
10. Kumar P, Islary B, Ramachandra R, Naik T. Axillary nerve schwannoma: A rare case report. Asian J Neurosurg. 2017. 12: 787-9
11. Liao H, Song W, Chen N, Chen F, Liu C, Lin F. Left lower lobe sleeve resection for endobronchial schwannoma. Ann Transl Med. 2019. 7: 50
12. Mrugala MM, Batchelor TT, Plotkin SR. Peripheral and cranial nerve sheath tumors. Curr Opin Neurol. 2005. 18: 604-10
13. Neurilemmoma (Schwannoma) Clinical Presentation: History and Physical Examination. Available from: https://www.emedicine.medscape.com/article/1256405-clinical [Last accessed on 2019 Nov 24].
14. Neurinoma-An Overview-Science Direct Topics. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/neurinoma [Last accessed on 2020 Apr 11].
15. Plexiform Neurofibroma-An Overview Science Direct Topics. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/plexiform-neurofibroma [Last accessed on 2020 Apr 11].
16. Pulmonary Malignant Peripheral Nerve Sheath Tumour- European Journal of Cardio-Thoracic Surgery. Available from: https://www.academic.oup.com/ejcts/article/46/2/331/358932 [Last accessed on 2020 Apr 11].
17. Roman M, Burbidge O, McCulloch T, Majewski A. Endobronchial benign nerve sheath tumour presenting with significant shortness of breath and haemoptysis. Oxf Med Case Reports. 2018. 2018: omy033
18. Schwannoma-Radiology. Available from: https://www.radiopaedia.org/articles/schwannoma?lang=us [Last accessed on 2020 May 18].
19. Sitenga JL, Aird GA, Nguyen A, Vaudreuil A, Huerter C. Clinical features and surgical treatment of schwannoma affecting the base of the tongue: A systematic review. Int Arch Otorhinolaryngol. 2017. 21: 408-13
20. Sun I, Pamir MN. Non-syndromic spinal schwannomas: A novel classification. Front Neurol. 2017. 8: 318
21. Takeda S, Miyoshi S, Minami M, Matsuda H. Intrathoracic neurogenic tumors-50 years’ experience in a Japanese institution. Eur J Cardiothorac Surg. 2004. 26: 807-12
22. Yukawa T, Shimizu K, Hirami Y, Okita R, Saisho S, Maeda A. A case report of intrapulmonary schwannoma. Gen Thorac Cardiovasc Surg. 2014. 62: 252-4