- Department of Neurology, University Hospital Cologne, Cologne, Germany
- Department of Stereotactic and Functional Neurosurgery, University Hospital of Cologne, Cologne, Germany.
Correspondence Address:
Hannah Jergas, Department of Neurology, University Hospital Cologne, Cologne, Germany.
DOI:10.25259/SNI_716_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hannah Jergas1, Juan C. Baldermann1, Jochen Wirths2, Michael T. Barbe1, Veerle Visser-Vandewalle2, Pablo Andrade2. Pallidal deep brain stimulation in a patient with nonketotic hyperglycemic hemichorea. 27-Jan-2023;14:24
How to cite this URL: Hannah Jergas1, Juan C. Baldermann1, Jochen Wirths2, Michael T. Barbe1, Veerle Visser-Vandewalle2, Pablo Andrade2. Pallidal deep brain stimulation in a patient with nonketotic hyperglycemic hemichorea. 27-Jan-2023;14:24. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12133
Abstract
Background: Hyperkinetic movement disorders secondary to brain tissue damage due to hyperglycemia are a rare complication of diabetes mellitus. Nonketotic hyperglycemic hemichorea (NH-HC) is characterized by a rapid onset of involuntary movements after increased serum glucose levels.
Case Description: We report on a case of a 62-year-old male patient with a 28-year history of Type II diabetes mellitus with NH-HC following an infect-associated exacerbation of blood glucose levels. Choreiform movements of the right upper extremity, face, and trunk persisted 6 months after onset. Due to failure of conservative treatments, we opted for unilateral deep brain stimulation of the globus pallidus internus, which led to complete cessation of symptoms within a week after initial programming. Symptom control was still satisfactory 12 months after surgery. No side-effects or surgery-associated complications were observed.
Conclusion: Globus pallidus internus DBS is an effective and safe treatment option for hyperkinetic movement disorders secondary to brain tissue damage caused by hyperglycemia. Postoperatively, stimulation effects can be observed quickly and effects persist even after 12 months.
Keywords: Deep brain stimulation, Diabetes mellitus, Globus pallidus, Hyperkinetic movement disorders, Nonketotic hyperglycemic hemichorea
INTRODUCTION
Hyperkinetic movement disorders secondary to brain tissue damage due to hyperglycemia are a rare complication of diabetes mellitus.[
CASE DESCRIPTION
A 62-year-old male patient with a 28-year history of Type II diabetes mellitus that was treated with metformin and empagliflozine was admitted to our clinic. Six months before first evaluation at our center, he experienced an episode of disturbed speech featuring dysarthria, dysphonia, and a weakness of the right-sided buccal branch of the facial nerve. On admittance to a local hospital, he was diagnosed with a urinary tract infection. Here, the admission diagnostic including noncontrast Computed tomograhpy (CT) followed by a CTperfusion and CT-angio excluded the possibility of a stroke. In addition, a serum glucose level of 500 mg/dL and Glycated hemoglobin (HbA1c) of 16.6% were found. T1 MRI revealed a hyperintense lesion within the left putamen and globus pallidus. Over the course of several weeks after antibiotic treatment for the infection and changes to antidiabetic medication, the patient developed progressive chorea of the upper right extremity. The previous unsuccessful therapeutic efforts included tiapride 600 mg/day and tetrabenazine 25 mg/day. Administration of higher doses was not possible as side effects occurred.
Relevant medical history included complications secondary to diabetes mellitus (e.g., peripheral neuropathy) and cardiovascular disease (e.g., arterial hypertension, coronary heart disease and ischemic cardiomyopathy). Five months after symptom onset, the patient experienced traumatic injury with serial rib fractures on the right hemithorax, as well as a periprosthetic fracture of the formerly replaced femur head, which left him wheelchair bound.
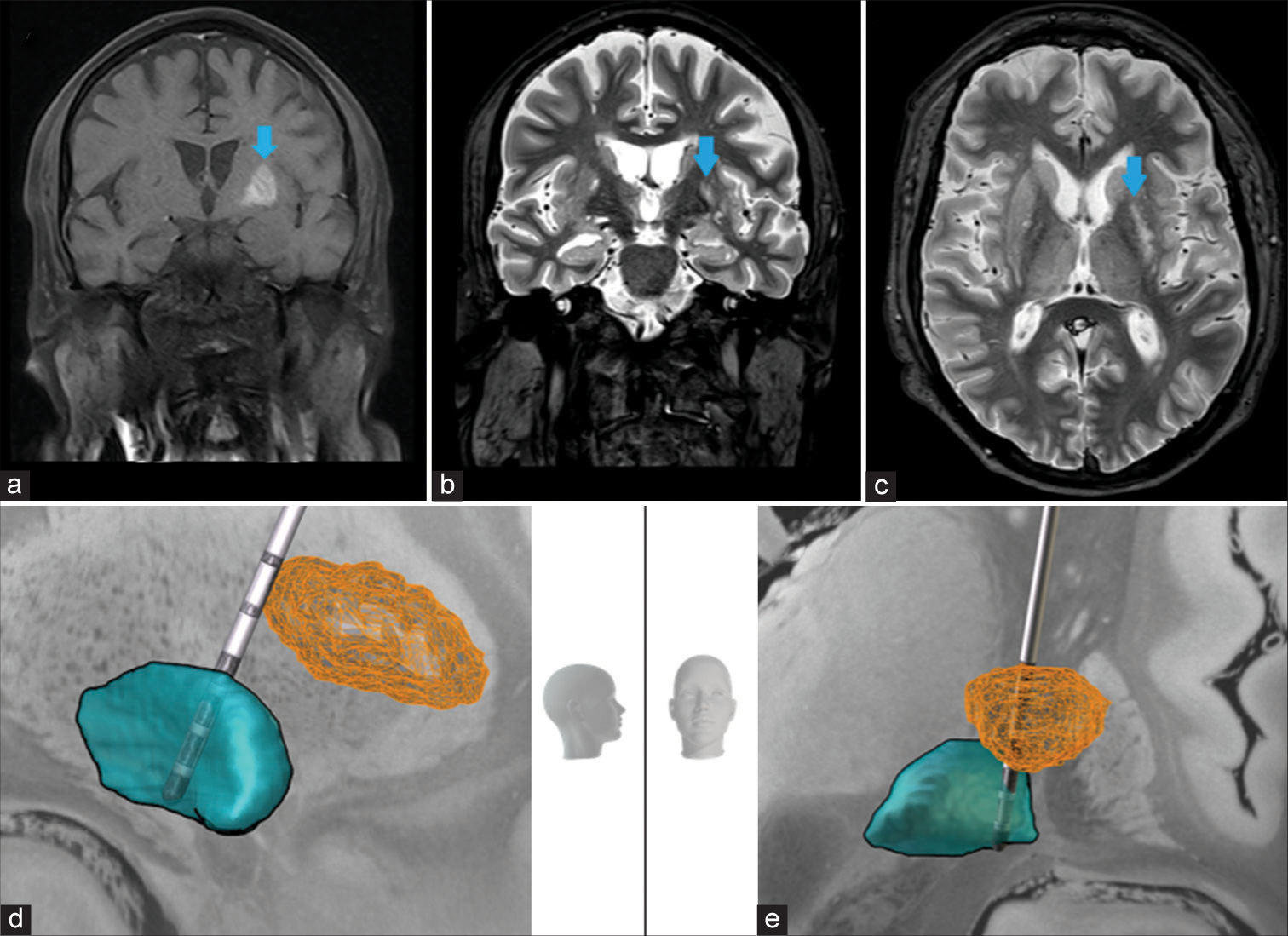
Neurological examination of the right-handed patient revealed ptosis and perioral synkinesis on the right face-side accompanying ballistic movements of the right arm and athetosis of the right hand without paresis, including involuntary movements of the trunk. He also reported hypesthesia of the right upper extremity. Images of the preoperative 3D-MRI revealing a hyperintense lesion in the left putamen which extends into the pallidum are shown in
Figure 1:
Results from preoperative MRI imaging and postoperative electrode localization. (a) shows a coronal section of the noncontrast T1-weighted MRI with the blue arrow pointing at the hyperintense lesion approximately 2 weeks after symptom onset (image by courtesy of Dr. J. Meyer, Radiologikum Krefeld) (b) coronal and (c) axial section of FLAIR-weighted 3 Tesla MRI used for stereotactic planning 6 months after symptom onset. The blue arrow points at the hyperintense lesion in (a), (b) and (c). Directional lead superimposed on a 7T MRI in the left GPi (blue) in relation to the lesion as extracted from preoperative imaging and normalized into Montreal Neurological Institute (MNI) space (orange) in (d) lateral view (e) anterior view. Head models indicate image orientation. 3D headmodel (“Head”;
The patient was implanted with a directional electrode (Cartesia D-Lead, Boston Scientific) unilaterally in the left GPi [
Supplementary Video
CONCLUSION
Our case shows that GPi DBS is an effective and safe treatment option for hyperkinetic movement disorders secondary to brain tissue damage caused by hyperglycemia. Postoperatively, stimulation effects can be observed quickly and effects persist even after 12 months.
Statement of ethics
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Data availability statement
The data that support the findings of this study are not publicly available due to patient privacy concerns but are available from the corresponding author on reasonable request.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Jochen Wirths received speaker’s honoraria from Boston Scientific. Michael T. Barbe received speaker’s honoraria from Medtronic, Boston Scientific, Abbott (formerly St. Jude), GE Medical, UCB, Apothekerverband Köln e.V. and Bial as well as research funding from the Felgenhauer-Stiftung, Forschungspool Klinische Studien (University of Cologne), Horizon 2020 (Gondola), Medtronic (ODIS), and Boston Scientific and advisory honoraria for the IQWIG. Veerle Visser-Vandewalle reports consultancies for Medtronic, Boston Scientific and St. Jude Medical. She received a grant from SAPIENS Steering Brain Stimulation. Pablo Andrade received speaker’s honoraria from Medtronic and Boston Scientific.
Video available online at:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY
References
1. Cardoso F, Seppi K, Mair KJ, Wenning GK, Poewe W. Seminar on choreas. Lancet Neurol. 2006. 5: 589-602
2. Cherian A, Thomas B, Baheti NN, Chemmanam T, Kesavadas C. Concepts and controversies in nonketotic hyperglycemia-induced hemichorea: Further evidence from susceptibility-weighted MR imaging. J Magn Reson Imaging. 2009. 29: 699-703
3. Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: A hyperviscosity syndrome?. Arch Neurol. 2002. 59: 448-52
4. Xiao F, Liu M, Wang XF. Involuntary choreiform movements in a diabetic patient. Lancet. 2019. 393: 1033