- Department of Neurosurgery, Roswell Park Cancer Institute, University at Buffalo, State University of New York, Buffalo, NY, USA
- Department of Neurosurgery, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA
- Department of Pathology, Roswell Park Cancer Institute, University at Buffalo, State University of New York, Buffalo, NY, USA
- Department of Radiation Medicine, Roswell Park Cancer Institute, University at Buffalo, State University of New York, Buffalo, NY, USA
- Department of Neuro oncology, Roswell Park Cancer Institute, University at Buffalo, State University of New York, Buffalo, NY
- Dent Neurologic Institute, Amherst, NY, USA
Correspondence Address:
Robert A. Fenstermaker
Department of Neurosurgery, Roswell Park Cancer Institute, University at Buffalo, State University of New York, Buffalo, NY, USA
Department of Neurosurgery, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA
DOI:10.4103/2152-7806.166782
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shakir HJ, Qiu J, Prasad D, Mechtler LL, Fenstermaker RA. Papillary tumor of the pineal region with extended clinical and radiologic follow-up. Surg Neurol Int 07-Oct-2015;6:
How to cite this URL: Shakir HJ, Qiu J, Prasad D, Mechtler LL, Fenstermaker RA. Papillary tumor of the pineal region with extended clinical and radiologic follow-up. Surg Neurol Int 07-Oct-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/papillary-tumor-of-the-pineal-region-with-extended-clinical-and/
Abstract
Background:Papillary tumor of the pineal region (PTPR) is a rare neoplasm with only anecdotal data to guide the treatment. Results of treatment with surgery, radiation therapy, and chemotherapy have been reported to have varying degrees of success. Here we report a patient with a PTPR, who underwent subtotal resection, gamma knife stereotactic radiosurgery, and adjuvant temozolomide chemotherapy.
Case Description:During 9 years of clinical and radiographic follow-up, the patient has had regression of residual tumor and remains asymptomatic.
Conclusion:When gross total resection of a PTPR is not possible, treatment with gamma knife stereotactic radiosurgery and temozolomide chemotherapy may provide long-term tumor control.
Keywords: Craniotomy, gamma knife, papillary tumor, pineal gland, radiosurgery, temozolomide
INTRODUCTION
Papillary tumor of the pineal region (PTPR), first described in 2003 by Jouvet et al.,[
CASE REPORT
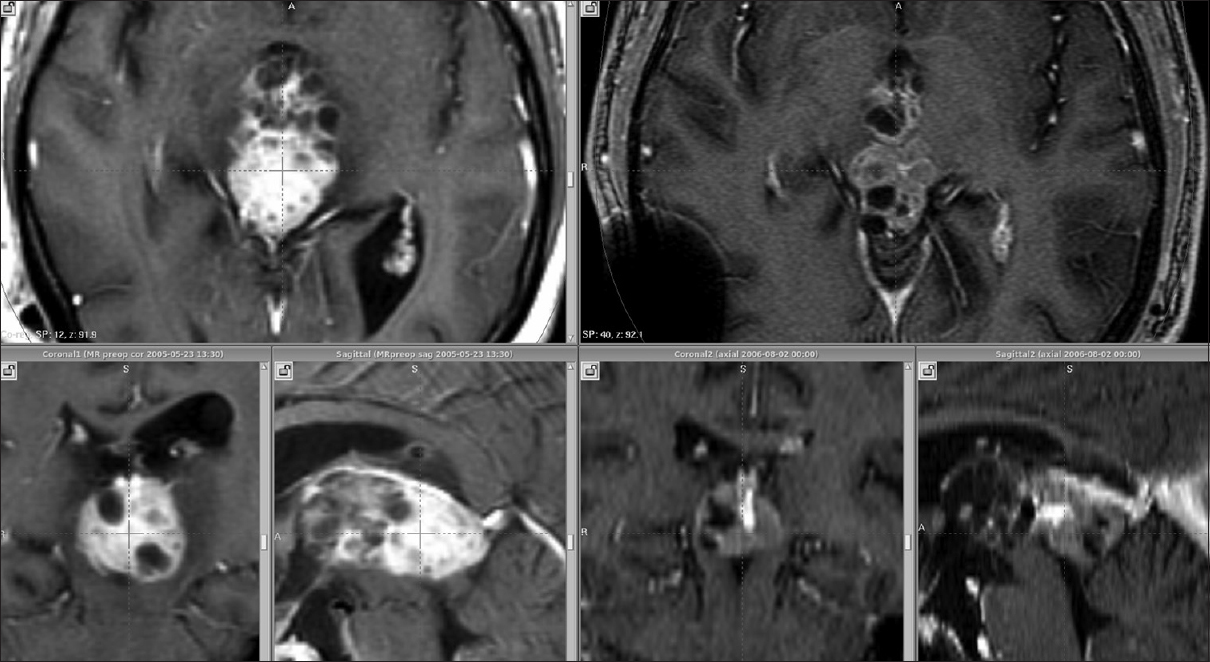
This 31-year-old man presented with a 2-year history of headaches, balance problems, intermittent double vision, and a single generalized seizure. Neurologic examination demonstrated bilateral papilledema, right hemiparesis, global cognitive impairment, wide-based gait, and slowness to initiate movements. Magnetic resonance imaging (MRI) revealed hydrocephalus and a posterior third ventricular tumor with a volume of 10.9 cm3 [
Figure 2
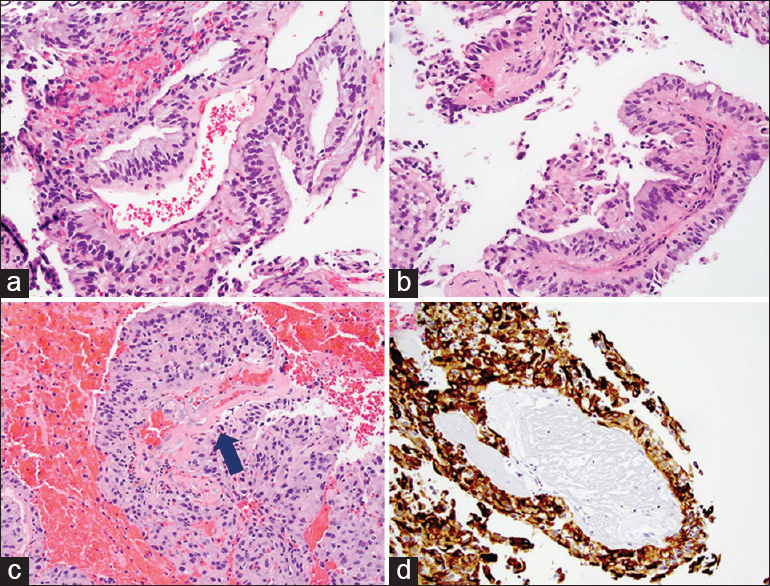
Representative H and E images (×200) of the tumor showing papillary architecture and columnar and cuboidal epithelial histology (a-c). There are hyalinized blood vessels (c, arrow) as well. There is no evidence of mitosis, necrosis, or vascular proliferation. Tumor cells are positive for cytokeratin CAM 5.2 (d, ×200)
Temozolomide was initiated postoperatively, and the patient received 12 cycles over a period of 1-year without the significant complications. Following this regimen, gamma knife stereotactic radiosurgery was performed using a dose of 18 Gy to the 50% isodose surface at the tumor margin. At the time of gamma knife treatment, residual tumor volume measured 4.2 cm3. The treatment provided 100% coverage of the tumor at the prescription dose with 85% selectivity and a gradient index of 2.75. Thereafter, an additional 12 cycles of temozolomide were given for a total of 24 cycles. At the conclusion of chemotherapy, tumor volume measured 3.5 cm3. Subsequently, the patient returned for semiannual MRI scans, which have shown continued tumor involution [
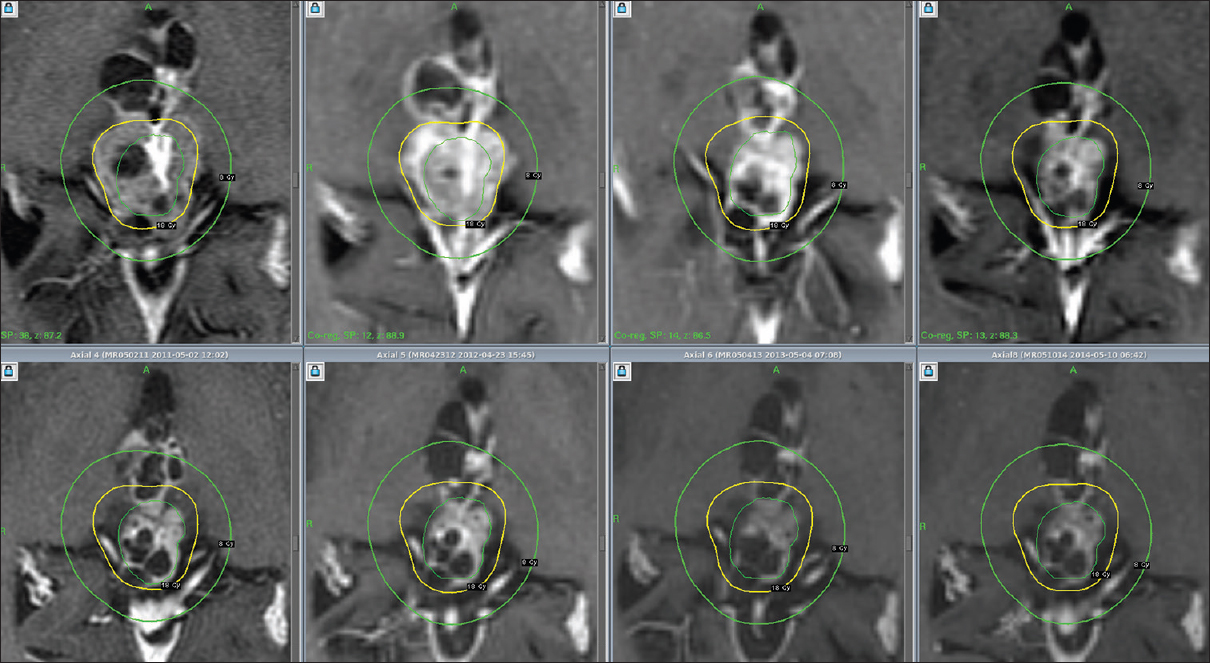
Figure 3
Serial T1-weighted coronal images beginning with day of gamma knife treatment and ending with current year (9 years after diagnosis) (upper set of images, left to right), and corresponding tumor volumes (lower set of images, also left to right) determined following co-registration of diagnostic images using Leksell GammaPlan 10 (Elekta, Henderson, NV, USA), with superimposition of the treatment plan on later image sets
DISCUSSION
Tumors of the pineal region are rare, accounting for 0.5–1.6% of all intracranial tumors.[
Little data concerning the combination of radiosurgery and chemotherapy have been reported. One group recently described a patient with PTPR in whom stereotactic radiosurgery was used as the sole treatment, leading to a gradual reduction in tumor size over a 5-year follow-up period.[
Our patient experienced a continued slow reduction in tumor volume over 9 years after initial surgical debulking, followed by chemotherapy with temozolomide and gamma knife radiosurgery midway through that regimen. In our patient, gamma knife radiosurgery was performed between cycles 12 and 13 of a chemotherapy regimen that totaled 24 cycles overall. Therefore, it is not strictly possible to separate the effects of gamma knife therapy from those of temozolomide therapy, with continued tumor volume reduction occurring both before and after gamma knife treatment. It is possible that a similar outcome could have been obtained with the radiosurgery alone, as may be seen with benign tumors such as vestibular schwannoma.
At present, treatment for PTPR involves a multispecialty approach with reliance upon maximum safe surgical resection and either fractionated radiation therapy or stereotactic radiosurgery.[
CONCLUSION
This report describes the long-term result of multimodal treatment in a single patient with PTPR. Following subtotal resection, gamma knife stereotactic radiosurgery, and temozolomide chemotherapy, this patient has experienced a continued slow decrease in tumor volume over time, even well after the conclusion of treatment. As additional cases are reported, the role of adjuvant therapies like stereotactic radiosurgery may become clearer. In the meantime, gamma knife radiosurgery, with or without temozolomide chemotherapy, should be considered as an option for the treatment of residual disease following subtotal resection of PTPR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Paul H. Dressel BFA for the preparation of the illustrations and Debra J. Zimmer for the editorial assistance.
References
1. Abela L, Rushing EJ, Ares C, Scheer I, Bozinov O, Boltshauser E. Pediatric papillary tumors of the pineal region: To observe or to treat following gross total resection?. Childs Nerv Syst. 2013. 29: 307-10
2. Boco T, Aalaei S, Musacchio M, Byrne R, Cochran E. Papillary tumor of the pineal region. Neuropathology. 2008. 28: 87-92
3. Dagnew E, Langford LA, Lang FF, DeMonte F. Papillary tumors of the pineal region: Case report. Neurosurgery. 2007. 60: E953-5
4. Fauchon F, Hasselblatt M, Jouvet A, Champier J, Popovic M, Kirollos R. Role of surgery, radiotherapy and chemotherapy in papillary tumors of the pineal region: A multicenter study. J Neurooncol. 2013. 112: 223-31
5. Fèvre-Montange M, Hasselblatt M, Figarella-Branger D, Chauveinc L, Champier J, Saint-Pierre G. Prognosis and histopathologic features in papillary tumors of the pineal region: A retrospective multicenter study of 31 cases. J Neuropathol Exp Neurol. 2006. 65: 1004-11
6. Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A. Papillary tumor of the pineal region. Am J Surg Pathol. 2003. 27: 505-12
7. Lorenzetti M, Motta F, Campanella R, Bauer D, Assi A, Arienta C. Adjuvant temozolomide chemotherapy for treatment of papillary tumor of the pineal region. World Neurosurg. 2011. 76: 160-3
8. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
9. Patel SK, Tomei KL, Christiano LD, Baisre A, Liu JK. Complete regression of papillary tumor of the pineal region after radiation therapy: Case report and review of the literature. J Neurooncol. 2012. 107: 427-34
10. Riis P, van Eck AT, Dunker H, Bergmann M, Börm W. Stereotactic radiosurgery of a papillary tumor of the pineal region: Case report and review of the literature. Stereotact Funct Neurosurg. 2013. 91: 186-9
11. Slavin KV, Ausman JI, Rengachary SS, Wilkins RH.editors. Tumors of the pineal region. Principles of Neurosurgery. London: Mosby; 1994. p. 29.1-29.16