- Departments of Neurosurgery, Puerta de Hierro University Hospital, Madrid, Spain.
- Departments of Internal Medicine, Puerta de Hierro University Hospital, Madrid, Spain.
- Neurophysiology, Puerta de Hierro University Hospital, Madrid, Spain.
Correspondence Address:
Ruth Prieto
Neurophysiology, Puerta de Hierro University Hospital, Madrid, Spain.
DOI:10.25259/SNI_20_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ruth Prieto, Alejandro Callejas-Díaz, Rasha Hassan, Alberto Pérez de Vargas, Luis Fernando López-Pájaro. Parvimonas micra: A potential causative pathogen to consider when diagnosing odontogenic brain abscesses. 06-Jun-2020;11:140
How to cite this URL: Ruth Prieto, Alejandro Callejas-Díaz, Rasha Hassan, Alberto Pérez de Vargas, Luis Fernando López-Pájaro. Parvimonas micra: A potential causative pathogen to consider when diagnosing odontogenic brain abscesses. 06-Jun-2020;11:140. Available from: https://surgicalneurologyint.com/surgicalint-articles/10068/
Abstract
Background: Brain abscess is a life-threatening entity which requires prompt and long-term antibiotic therapy, generally associated with surgical drainage, and eradicating the primary source of infection. Parvimonas micra (Pm) has only been reported once before as the lone infecting organism of an orally originated, solitary brain abscess. Diagnosing brain abscesses caused by this Gram-positive anaerobic coccus, constituent of the oral cavity flora, is challenging, and an optimal treatment regimen has not been well established. We report the diagnosis and successful treatment of a Pm caused odontogenic brain abscess.
Case Description: A 62-year-old immunocompetent male with a right-parietal brain abscess presented with headache and seizures. He was started on empirical antibiotic therapy and subsequently underwent surgical drainage. The only source of infection found was severe periodontitis with infected mandibular cysts. Thus, tooth extraction and cyst curettage were performed 1 week after brain surgery. Cultures of brain abscess fluid were negative, but amplification of bacterial 16S ribosomal RNA (rRNA) with polymerase chain reaction demonstrated Pm. After 3 weeks of intravenous ceftriaxone and metronidazole, the patient was switched to oral metronidazole and moxifloxacin for 6 weeks.
Conclusions: This case highlights the potential risk of untreated dental infections causing brain abscesses. Pm should be considered as a possible pathogen of odontogenic brain abscesses despite its presence usually not being detected by standard bacterial cultures. Therefore, 16S rRNA gene sequencing analysis is strongly recommended for bacterial identification before defining brain abscesses as cryptogenic.
Keywords: 16S rRNA analysis, Brain abscess, Dental infection, Odontogenic abscess, Parvimonas micra
INTRODUCTION
Brain abscess is one of the most serious infections of the central nervous system. It consists of a focal purulent collection of the brain parenchyma.[
Pm, formerly known as Peptostreptococcus micros and Micromonas micros, is a Gram-positive anaerobic coccus that is normally found in the human flora of the oral cavity and gastrointestinal tract.[
CASE DESCRIPTION
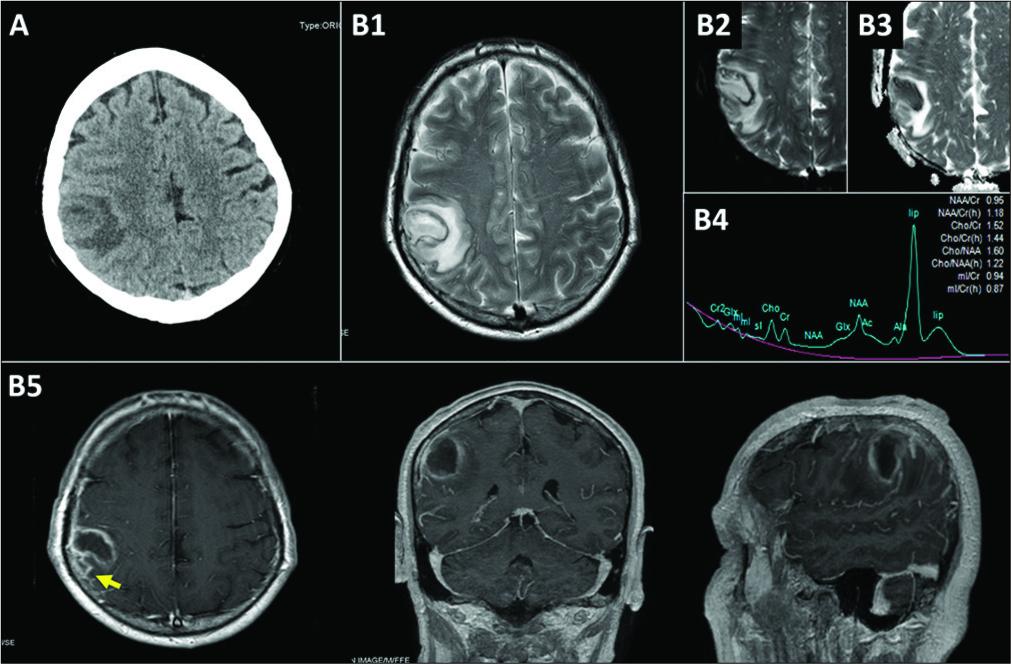
A 62-year-old male presented to the hospital with a 1-week history of headache and an episode of transitory paresthesia in the left upper limb followed by a 2-min lapse in awareness and a claw-like left hand position highly suggestive of an atypical absence seizure. He had no history of fever, malignancy, diabetes mellitus, or corticosteroid use. Laboratory results revealed a normal white blood cell count and a moderately elevated C-reactive protein (63.6 mg/L–normal levels are below 10 mg/L–). The patient was alert and presented neither neurological deficits nor signs of meningeal irritation. An urgent brain computed tomography (CT) scan demonstrated an expansive process in the right parietal lobe [
Figure 1:
Preoperative neuroradiological studies. (A) Computed tomography scan showing an isodense 3-cm-parietal mass with surrounding digitiform edema, (B) preoperative magnetic resonance imaging (MRI). (B1) T2-weighted MRI showing a hyperintense lesion with significant perilesional edema, (B2) diffusion-weighted imaging MRI showing a bright signal within the lesion (restriction of water diffusion), (B3) low apparent diffusion coefficient value within the lesion fluid. (B4) MR spectroscopy showing a high lipid level (lip) and decreased N-acetylaspartate, (B5) gadolinium- enhanced T1-weighted MRI showing a low-intensity lesion with rim enhancement which extended to the subarachnoid space (arrow).
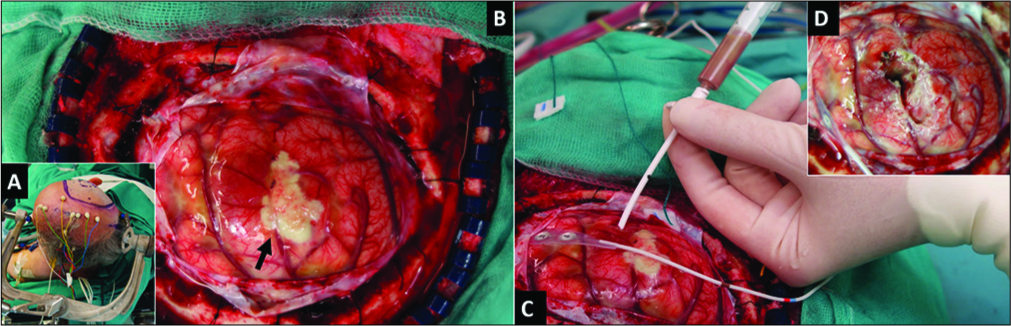
On day 3, the patient underwent craniotomy with neurophysiological monitoring given the proximity of the lesion to the motor cortex. After opening the dura mater, a swollen brain was found [
Figure 2:
Intraoperative photographs. (A) The patient was placed in the lateral position, (B) following dura opening, a swollen brain was found with a whitish subarachnoid area (arrow), (C) a catheter was inserted at the point where the abscess capsule was closest to the cortex. Brownish liquid pus was aspirated for mass decompression and later microbiological study. (D) Surgical view following removal of the whitish subarachnoid area and cavity washing.
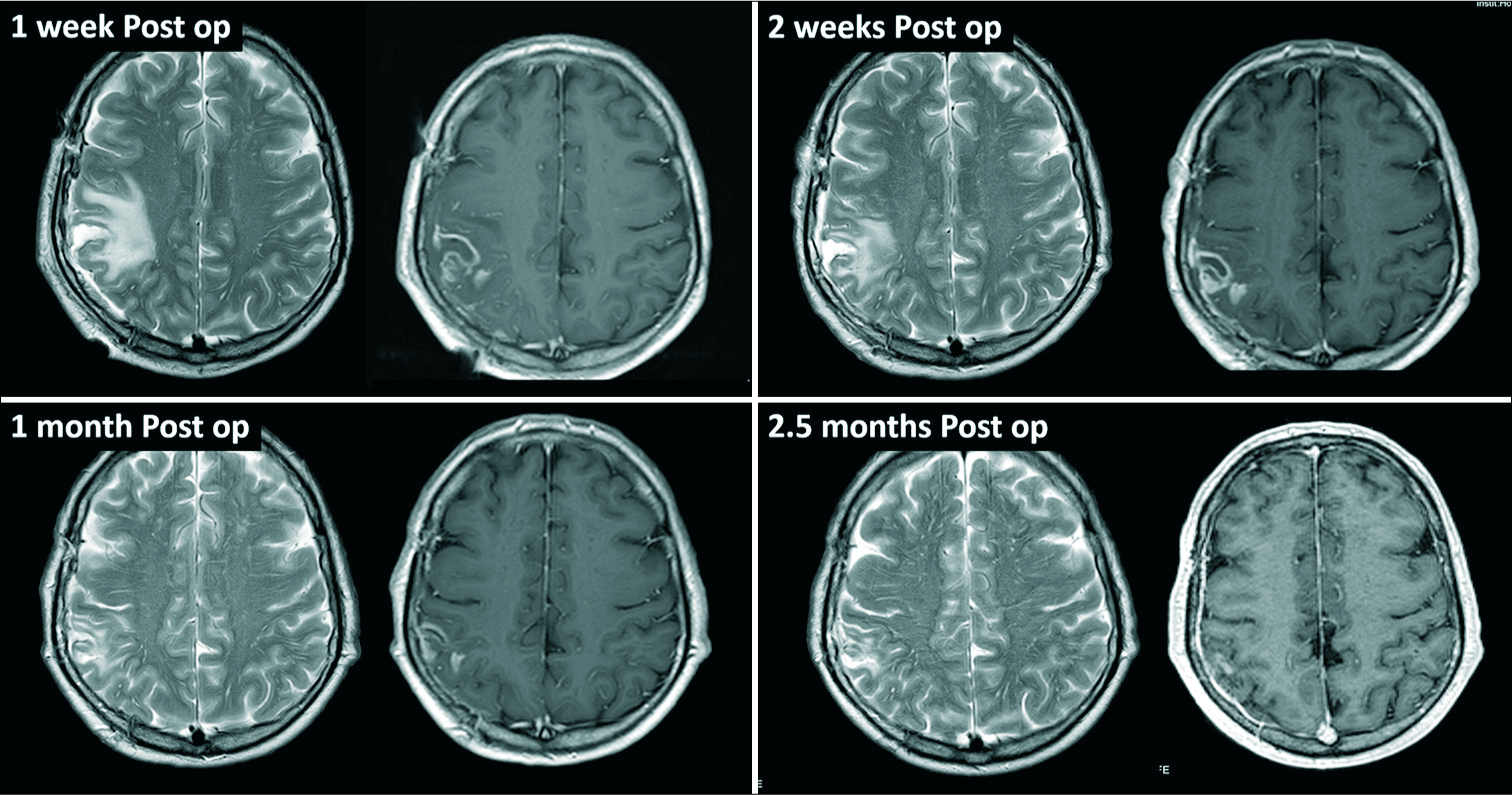
The patient was also assessed by maxillofacial surgeons who detected severe periodontitis with infected mandibular cysts. This condition was considered the primary source of brain infection, and 1 week after craniotomy the patient underwent extraction of the lower teeth and curettage of mandibular cysts. Unfortunately, the material of the extracted teeth was not sent for microbiological studies. The patient was discharged in excellent conditions after 3 weeks of intravenous antibiotics, followed by 1 week of oral metronidazole and moxifloxacine. Oral antibiotics were maintained for 5 additional weeks. Follow-up MRIs demonstrated favorable progress without any radiological suspicion of recurrence [
DISCUSSION
The seriousness of brain abscesses requires prompt joint action by neurosurgeons and infectious-disease specialists. Merely focusing on the focal suppurative process of the brain parenchyma is not enough for a successful treatment. It is paramount to identify and eradicate the primary source of infection and the specific causative microorganism. In the case presented, Pm isolated in the brain abscess was assumed to have an oral origin based on the evident signs of dental infection. Abscess location in the parietal lobe supports that hematogenous dissemination is the major pathophysiological mechanism of brain abscess of odontogenic origin.[
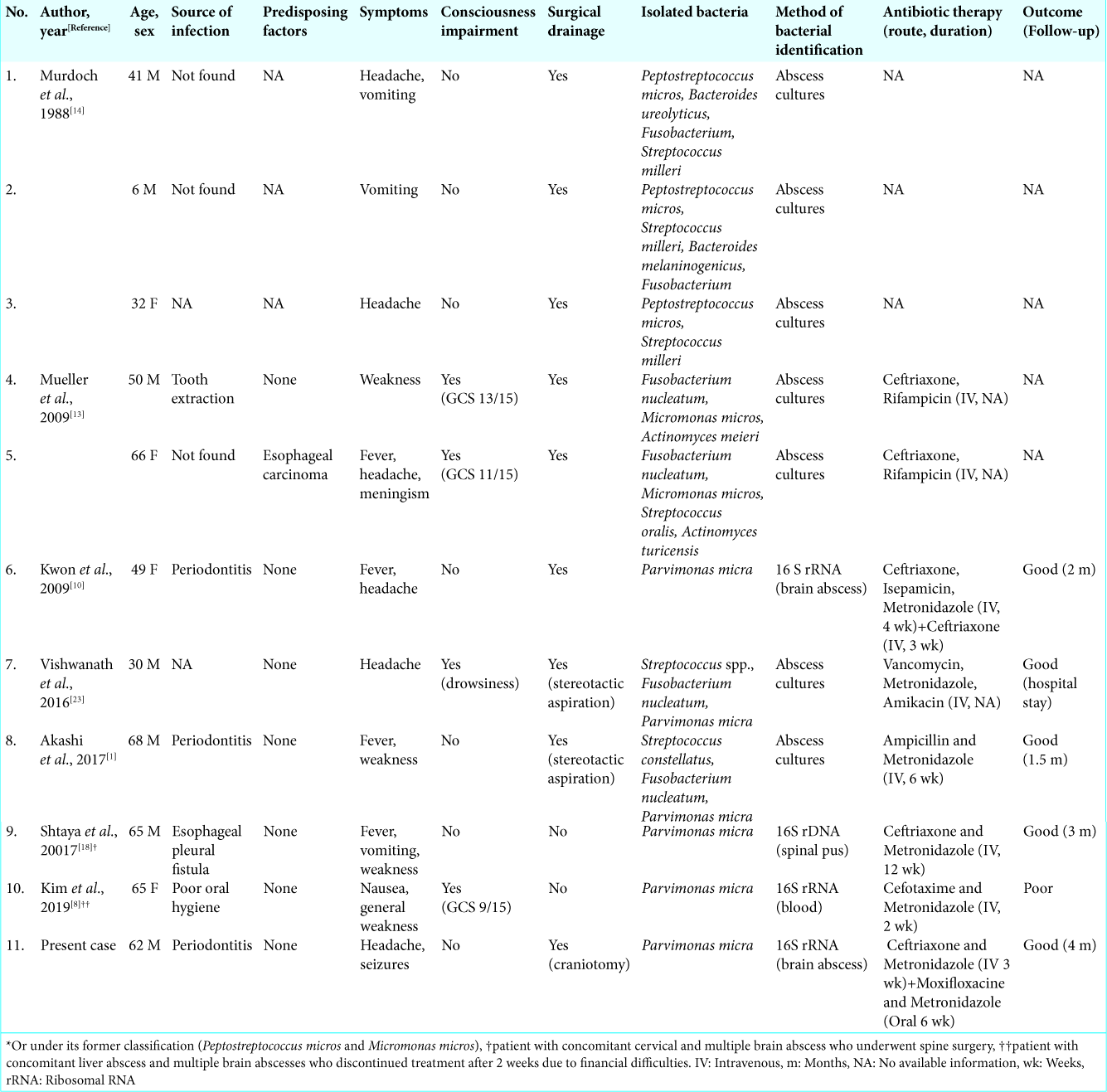
After a thorough literature review, we found only ten previous cases of brain abscesses in which Pm, an organism typically found in the human oral flora, was isolated [
Optimal treatment of intracranial Pm infections remains uncertain.[
CONCLUSIONS
We report the successful diagnosis and treatment of a solitary brain abscess caused by Pm in a patient with severe periodontal infection. This case highlights the potential risk of untreated dental infections, which may lead to life- threating brain abscesses even in healthy patients. This report also supports that 16S rRNA analysis is a valuable technique to detect Pm in cases with culture-negative brain abscesses.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are indebted to Crystal Smith and Liliya Gusakova, reference librarians of the National Library of Medicine, National Institutes of Health (Bethesda, MD, USA), and to Cristina Escudero and Ana Puerto, librarians of Puerta de Hierro University Hospital (Madrid, Spain) for their generous assistance during the process of retrieving some of the original articles used in this study. We are also grateful to George Hamilton for his critical review of the language and style of the manuscript.
References
1. Akashi M, Tanaka K, Kusumoto J, Furudoi S, Hosoda K, Komori T. Brain abscess potentially resulting from odontogenic focus: Report of three cases and a literature review. J Maxillofac Oral Surg. 2017. 16: 58-64
2. Akhaddar A.editors. Brain abscesses. Atlas of Infections in Neurosurgery and Spinal Surgery. Berlin, Germany: Springer; 2017. p. 73-94
3. Cobo F, Rodriguez-Granger J, Sampedro A, Aliaga-Martinez L, Navarro-Mari JM. Pleural effusion due to Parvimonas micra A case report and a literature review of 30 cases. Rev Esp Quimioter. 2017. 30: 285-92
4. Corson MA, Postlethwaite KP, Seymour RA. Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis. 2001. 7: 61-5
5. Ewald C, Kuhn S, Kalff R. Pyogenic infections of the central nervous system secondary to dental affections-a report of six cases. Neurosurg Rev. 2006. 29: 163-7
6. García-Sánchez JE, Fresnadillo MJ, García-Sanchez E. New anaerobic bacterial species in human infections. Enferm Infecc Microbiol Clin. 2010. 28: 173-84
7. George IA, Pande A, Parsaei S. Delayed infection with Parvimonas micra following spinal instrumentation. Anaerobe. 2015. 35: 102-4
8. Kim EY, Baek YH, Jung DS, Woo KW. Concomitant liver and brain abscesses caused by Parvimonas Micra. Korean J Gastroenterol. 2019. 73: 230-4
9. Ko JH, Baek JY, Kang CH, Lee WJ, Lee JY, Young S. Bacteriemic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe. 2015. 34: 161-3
10. Kwon O, Uh Y, Jang IH, Lee HG, Yoon KJ, Kim HY. A case of brain abscess due to Parvimonas micra. Korean J Clin Microbiol. 2009. 12: 129-32
11. Lee Y, Park Y, Kim MS, Young D, Jeong SH, Lee K. Antimicrobial susceptibility patterns for recent clinical isolates of anaerobic Bacteria in South Korea. Antimicrob Agents Chemother. 2010. 54: 3993-397
12. Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M. Intracranial bacterial infections of oral origin. J Clin Neurosci. 2015. 22: 800-6
13. Mueller AA, Saldamli B, Stübinger S, Walter C, Flückiger U, Merlo A. Oral bacterial cultures in nontraumatic brain abscesses: Results of a first-line study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 107: 469-76
14. Murdoch DA, Mitchelmore IJ, Tabaqchali S. Peptostreptococcus micros in polymicrobial abscesses. Lancet. 1988. 12: 594-
15. Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain abscess: Management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg. 2011. 75: 716-26
16. Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect. 2006. 53: 221-7
17. Prieto R, Ortega C. Parafalcine subdural empiema: The unresolved controversy over the need for surgical treatment. Surg Neurol Int. 2019. 10: 203-
18. Shtaya A, Schuster H, Riley P, Harris K, Hettige S. Oesophageal pleural fistula presenting with Parvimonas micra infection causing cervical and brain abscesses. Anaerobe. 2017. 47: 233-7
19. Sudhaharan S, Chavali P, Vemu L. Anaerobic brain abscess. Iran J Microbiol. 2016. 8: 120-4
20. Tindall BJ, Euzeby JP. Proposal of Parvimonas gen. Nov. and Quatrionicoccus gen. Nov. As replacement for illegitimate, prokaryotic, generic names Micromonas Murdoch and Shah 2000 and Quadricoccus Maszenan et al 2002 respectively. Int J Syst Evol Microbiol. 2006. 56: 2711-3
21. Uemura H, Hayakawa K, Shimada K, Tojo M, Nagamatsu M, Miyoshi-Akiyama T. Parvimonas micra as a causative organismo of spondylodiscitis: A report of two cases and a literature review. Int J Infect Dis. 2014. 23: 53-5
22. Veloo AC, Welling GW, Degener JE. Antimicrobial susceptibility of clinically relevant gram-positive anaerobic cocci collected over a three-year period in the Netherlands. Antimicrob Agents Chemother. 2011. 55: 1199-203
23. Vishwanath S, Shenoy PA, Gupta A, Menon G, Chawla K. Brain abscess with anaerobic gram-negative Bacilli Case series. J Case Rep. 2016. 6: 467-74