- Spine unit, Department of Orthopedic and Traumatology, Clínica Alemana, Santiago, Chile,
- Department of Orthopedic and Traumatology, School of Medicine, University del Desarrollo, Santiago, Chile.
Correspondence Address:
Ratko Yurac
Spine unit, Department of Orthopedic and Traumatology, Clínica Alemana, Santiago, Chile,
Department of Orthopedic and Traumatology, School of Medicine, University del Desarrollo, Santiago, Chile.
DOI:10.25259/SNI_253_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ratko Yurac1,2, Alvaro Silva1,2, Matias Delgado1, Marilaura Nuñez2, Juan Lopez2, Bartolome Marre1,2. Pathological axis fracture secondary to a solitary bone plasmacytoma: Two cases and a literature review. 14-Apr-2021;12:165
How to cite this URL: Ratko Yurac1,2, Alvaro Silva1,2, Matias Delgado1, Marilaura Nuñez2, Juan Lopez2, Bartolome Marre1,2. Pathological axis fracture secondary to a solitary bone plasmacytoma: Two cases and a literature review. 14-Apr-2021;12:165. Available from: https://surgicalneurologyint.com/surgicalint-articles/10708/
Abstract
Background: Solitay bone plasmocytoma (SBP) account for just 5–10% of all plasma cell neoplasms. They are infrequent in the cervical spine, especially involving the C0–C2 segment. In this article we conducted a literature review and present the diagnosis, management and long term course of two patients with SBP of C2 causing cervical instability.
Methods: We assessed the clinical records of two patients with SBP in C2 and cervical instability attributed to SP-B involving C2. Both patients presented with progressive, severe cervicalgia, and the “sensation” of skull instability. Magnetic resonance imaging revealed an extensive, infiltrative lesion involving C2 vertebral body and lateral masses, consistent with a plasmacytoma.
Results: Both patients underwent emergency posterior surgical stabilization with craniocervical fixation; this was accompanied by a C2 transpedicular biopsy. Postoperatively, patients exhibited no focal neurological deficits and rapidly became pain free. They additional recieved 25 sessions of local conventional radiation therapy. Both patients are doing well as respective 2 and 7-year follow-up.
Conclusion: Although rare, unstable SBP may present atypical cervical location that readily responds to surgical descompression/fusion and radiotherapy.
Keywords: Bone cyst, Cervical vertebrae, Multiple myeloma, Solitary plasmacytoma
INTRODUCTION
Solitary plasmacytoma (SP) is a rare hematological malignancy characterized by the localized proliferation of neoplastic monoclonal plasma cells, in the absence of multiple myeloma (MM), with <10% infiltration of plasma cells into the bone marrow.[
SBP is infrequent in the cervical spine, accounting for about 8% of cases[
We reviewed two patients with SBP at C2 who presented with severe axial neck pain and craniocervical instability treated with posterior descompression/stabilization and postoperative radiotherapy.
Case 1
A 73-year-old female presented with 1.5 months of progressive, severe cervicalgia, and a C2 bilateral root neurological deficit.
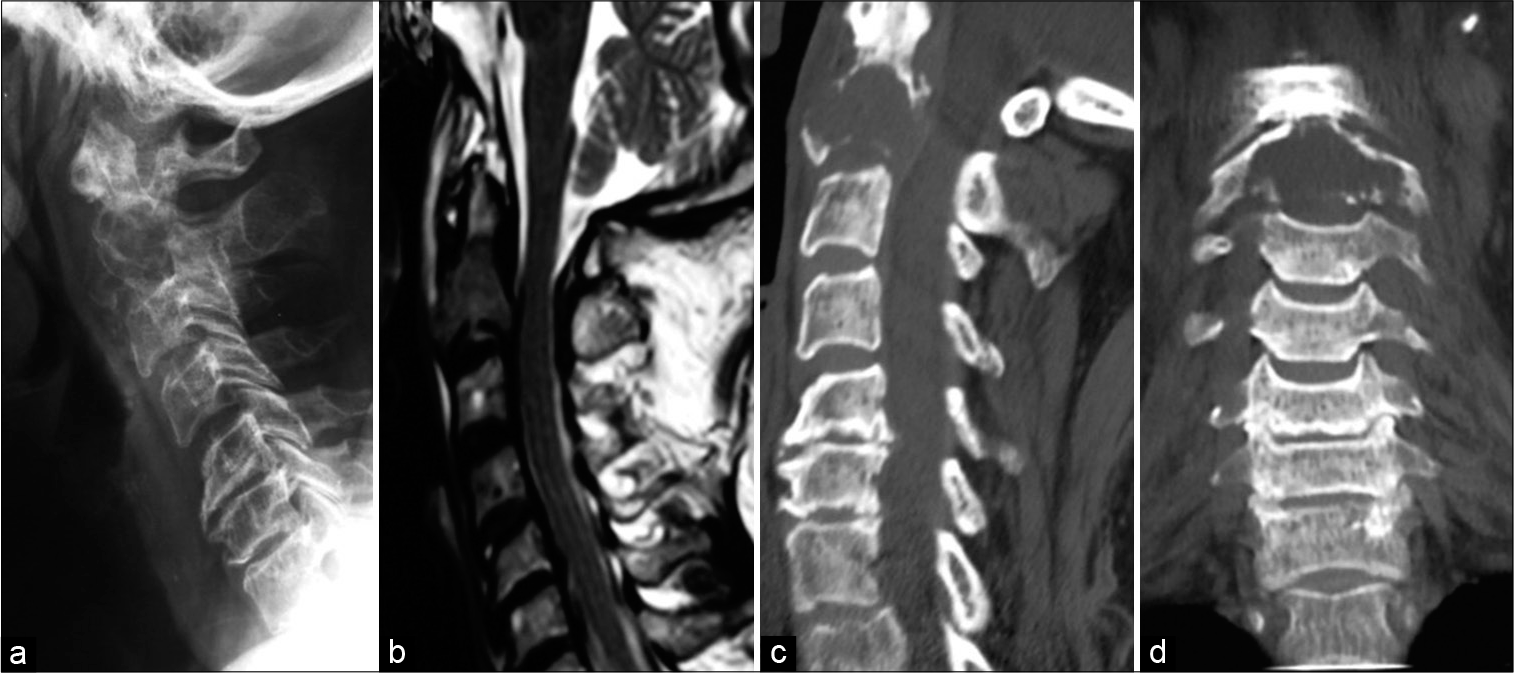
Cervical X-rays revealed a phatologic fracture of C2 secondary to a vertebral body lytic lesion. CT scan showed the lytic lesion involving both lateral mases of C2 with pathological fracture. Magnetic resonance (MR) showed partial epidural compromise [
Figure 1:
Case 1: Preoperative imaging. (a) Radiographs show a radiolucent C2 vertebral body, (b) T2-weighted magnetic resonance imaging (MRI) reveals hypointensity of the C2 vertebral body, (c and d) sagittal and coronal CT scan reveals instability due to severe lytic and destructive lesion in the vertebral body at C2.
Surgery
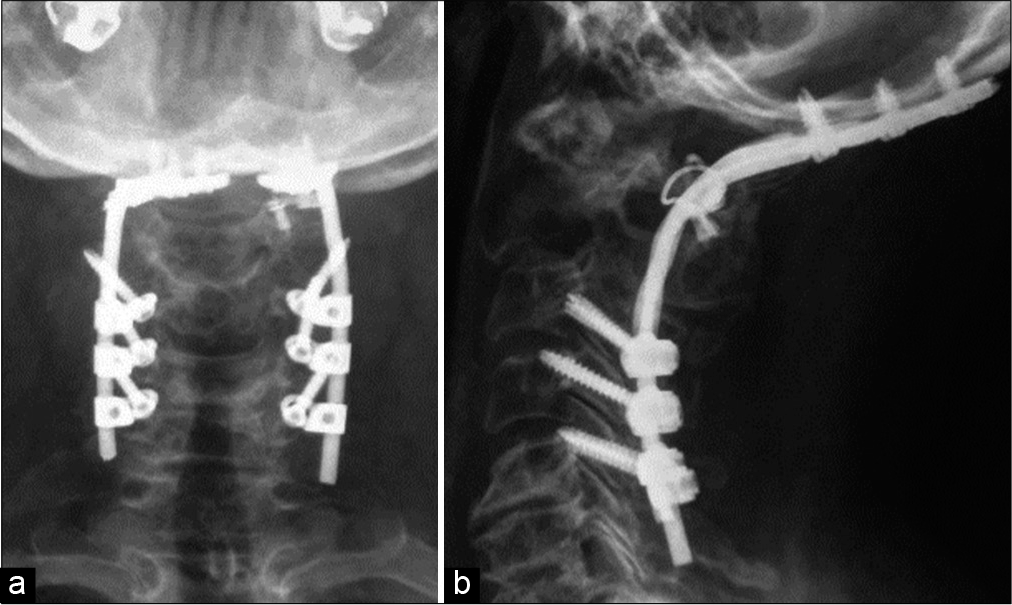
Posterior C0-C5 fusion with craniocervical fixation under halo traction was performed accompanied by the placement of iliac crest autograft (i.e., using the CerviFix rod system Synthes®; Stratec Medical, 4436 Oberdorf, Switzerland) accompanied by the placement of iliac crest autograft. Intraoperatively, anterior displacement of C0–C1 over C2 required titanium braided wires to achieve reduction. An additional posterior transpedicular biopsy was performed of C2 [
Histopathology
Histopathological and immunohistochemical studies of the lesion were consistent with a plasmacytoma (i.e., the diagnosis of SP-B was confirmed). Later, the patient underwent conventional radiotherapy (RT) (25 sessions [50 Gy]) with complete remission. Seven years later, the patient remains intact, without any evidence of recurrent disease.
Case 2
A 56-year-old male presented with 1 month of progressive, severe posterior neck pain and craniocervical paresthesias.
CT scan revealed an extensive, infiltrative C2 vertebral lesion that compromised vertebral body, the lower third of odontoid process, both lateral masses, pedicles and facet processes, and part of the axis laminae. The lesion had a low signal intensity on T1 WI MR, high signal intensity on T2 and STIR WI MR and irregularly enhanced contrast images without epidural extension [
Figure 3:
Case 2: Preoperative imaging. (a) CT scan shows listhesis and instability due to an infiltrative, destructive lesion in the vertebral body at C2, (b) T2-weighted MRI reveals hiperintensity of the C2 vertebral body, (c and d) MRI shows contrast enhancement in vertebral body, pedicles and the C2 laminae.
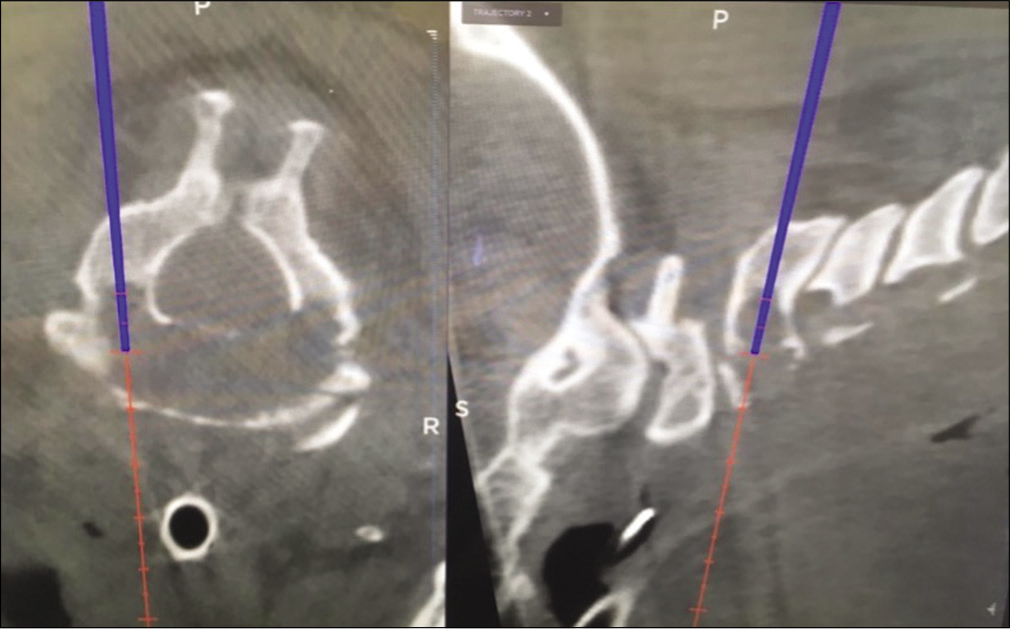
Surgery
Surgery was performed under neuromonitoring and neuronavigation support and included a bone biopsy through the C2 pedicles [
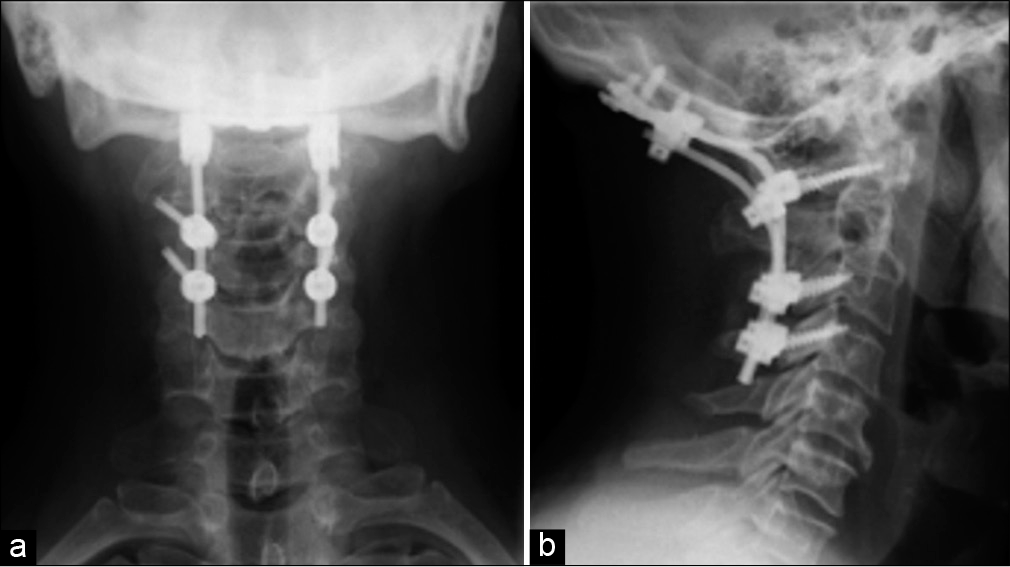
Postoperative course
Within 1 day, the patient had no pain or neurological deficits. The myelogram was normal, and postoperative images documented good implant alignment and position. He was discharged on the 5th postoperative day with a Miami J collar. Histopathological and immunohistochemical studies of the lesion were consistent with the diagnosis of a SP-B.
Conventional RT (25 sessions: 45 Gy) was begun in the 6th postoperative week. Three months later, the whole-body PET CT revealed adequate local control, without other bone lesions and good alignment. However, at the current time, the clinical course appears consistent with smoldering MM, including a slight decrease in light chains.
Nevertheless, 21 months later, the cervical CT and PET showed an intact patient with good local control without recurrence of the C2 lesion.
DISCUSSION
SBP accounts for just 3% of all plasma cell neoplasms.[
Approximately two-thirds of SBP eventually progress to MM within 1.75–4 years of diagnosis.[
The current international myeloma working group criteria for SBP are (1) a solitary bone or soft tissue lesion, verified by bone biopsy with evidence of clonal plasma cells; (2) normal bone marrow, without evidence of plasma cells or, failing that, <10% involvement; (3) no evidence of bone lesions other than the primary solitary lesion on MRI and/or CT of the spine and pelvis; and (4) the absence of any target organ damage (e.g., hypercalcemia, renal failure, and anemia).[
Tissue biopsy and histological and immunohistochemical findings, identifying the presence of a homogeneous infiltrate of monoclonal plasma cells, make it possible to establish the initial diagnosis.[
Radiation therapy is first-line treatment for SBP in many patients,[
Despite the high rate of local control with RT and/or surgery, rates for tumor recurrence and progression to MM are high.[
CONCLUSION
SBP involving the C2 cervical vertebral body is very rare but may be successfully managed with biopsy, decompression, and fusion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmadi SA, Slotty PJ, Munoz-Bendix C, Steiger HJ, Cornelius JF. Early surgical occipitocervical stabilization for plasma cell neoplasms at the craniocervical junction: Systematic review and proposal of a treatment algorithm. Spine J. 2016. 16: 91-104
2. Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European expert panel. J Hematol Oncol. 2018. 11: 10
3. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone extramedullary plasmacytoma, and multiple myeloma: Incidence and survival in the United States, 1992-2004. Br J Haematol. 2009. 144: 86-94
4. Gossios K, Argyropoulou M, Stefanaki S, Fotopoulos A, Chrisovitsinos J. Solitary plasmacytoma of the spine in an adolescent: A case report. Pediatr Radiol. 2002. 32: 366-9
5. Huang W, Cao D, Ma J, Yang X, Xiao J, Zheng W. Solitary plasmacytoma of cervical spine: Treatment and prognosis in patients with neurological lesions and spinal instability. Spine (Phila Pa 1976). 2010. 35: E278-84
6. Jawad MU, Scully SP. Skeletal plasmacytoma: Progression of disease and impact of local treatment; an analysis of SEER database. J Hematol Oncol. 2009. 2: 41
7. Mheidly K, De La Chapelle TL, Hunault M, Benboubker L, Benchalal M, Moreau P. New insights in the treatment of patients with solitary bone plasmacytoma. Leuk Lymphoma. 2019. 60: 2810-3
8. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014. 15: e538-48
9. Salaun PY, Gastinne T, Frampas E, Bodet-Milin C, Moreau P, Bodéré-Kraeber F. FDG-positron-emission tomography for staging and therapeutic assessment in patients with plasmacytoma. Haematologica. 2008. 93: 1269-71
10. Shen X, Liu S, Wu C, Wang J, Li J, Chen L. Survival trends and prognostic factors in patients with solitary plasmacytoma of bone: A population-based study. Cancer Med. 2020. 10: 462-70
11. Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol). 2004. 16: 405-13