- Department of Neurosurgery, Xingtai People’s Hospital Hebei Medical University, Xingtai, China,
- Department of Neurosurgery, Medical Faculty, Heinrich-Heine-University, Düsseldorf, Germany,
- Department of Pathology, Xingtai People’s Hospital Hebei Medical University,
- Department of Microbiology and Immunology, Xingtai Medical College, Xingtai, China,
- Department of Neurosurgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Correspondence Address:
Sajjad Muhammad, Department of Neurosurgery, Medical Faculty, Heinrich-Heine-University, Düsseldorf, Germany; Department of Neurosurgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
DOI:10.25259/SNI_791_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rui Zhang1,2, Xin Xu3, Huakang Zhou2, Dongying Yao3, Ru Wei4, Sajjad Muhammad2,5. Pediatric angiocentric glioma with acute intracerebral hemorrhage: A case report with 36 months follow-up. 06-Oct-2021;12:499
How to cite this URL: Rui Zhang1,2, Xin Xu3, Huakang Zhou2, Dongying Yao3, Ru Wei4, Sajjad Muhammad2,5. Pediatric angiocentric glioma with acute intracerebral hemorrhage: A case report with 36 months follow-up. 06-Oct-2021;12:499. Available from: https://surgicalneurologyint.com/surgicalint-articles/11161/
Abstract
Background: Angiocentric glioma (AG) is an extremely rare intracranial tumor that was first described in 2005 and identified as a special type of intracranial tumor in 2007 by the WHO, which mainly affects children and young adolescents. Epilepsy is the main presentation; therefore, it was recognized as a seizure-related tumor in the past. Here, we report a case of AG with acute intracerebral hemorrhage (ICH) as the first symptom who never had a seizure onset.
Case Description: A 3-year-old girl with the right limb weakness was admitted to our hospital 4 h after onset in 2018. Computed tomography showed a hematoma of about 20 ml accompanied by a hyper/iso-dense spheroid lesion located in the sub-cortex of the left parietal lobe. Magnetic resonance image (MRI) showed signs of hypointense signal in T1, T2, and fluid-attenuated inversion recovery sequence, distinct enhancement of this tumefactive lesion in the contrast-enhanced sequence. Thus, the admission diagnosis was neoplasm with acute ICH. A gross total resection of the tumor was achieved by parietal craniotomy. The histopathological diagnosis was AG. No signs showed tumor recurrence after 36 months of follow-up.
Conclusion: This is the sole case of AGs with acute intracranial hemorrhage as the first symptom without any kind of epilepsy by far. This case had unique MRI signs that were different from the previous description. This case enriches the clinical and radiological manifestations of AG and reveals that further investigations are needed to further understand AG.
Keywords: Angiocentric glioma, Epilepsy, Intracranial hemorrhage
INTRODUCTION AND HISTORICAL BACKGROUND
Angiocentric glioma (AG) is a rare intracranial tumor. WANG and Lellouch-Tubiana, respectively, 1st time reported in 2005. This special type of tumor mainly affects children and young adults.[
Two years later, the WHO officially confirmed it as a central nervous system entity tumor for the 1st time in the classification of tumors of the central nervous system.[
Mostly AG is located in the supratentorial area in hemispheric lobes, with a benign biological behavior: slow-growing, possibility to be surgically removed, rare postoperation recurrence, and low proliferation index; so AG was classified as WHO Grade I.[
CLINICAL PRESENTATION
A 3-year-old girl was admitted to our emergency department due to the sudden onset of the right limb weakness.
Physical examination showed a conscious child with poor spirit, normal-sized bilateral reactive pupils, hemiplegia of the right limb with low muscle tone, and muscle strength level 0. The child had vomited several times after the onset. No seizures occurred before and after onset.
Diagnosis
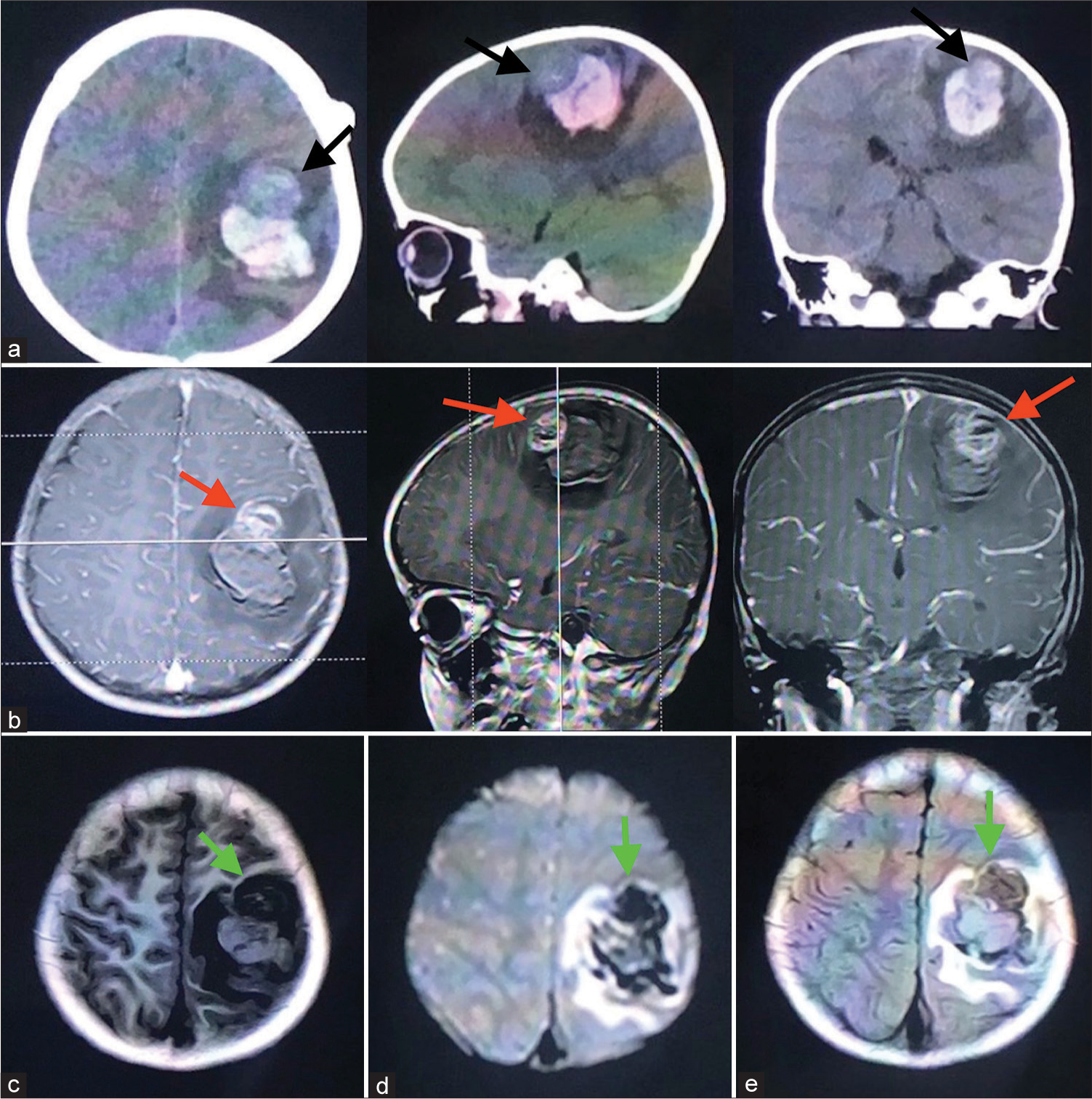
After admission, a computed tomography (CT) scan of the head showed a hematoma with the amount of 20 ml in the left parietal lobe. The primary diagnosis was ICH. However, a suspect lesion has a different density anterior to the hematoma suggesting the presence of neoplasm. The MRI scan and MRI contrast enhancement showed that admix signals (hypointense of the tumor part and isointense of the hematoma part) on T1 sequence, hypointense or isointense lesion with annularity hyperintense around on T2 and FLAIR sequence, and part of the lesion can be significantly enhanced on the contrast sequence. Thus, the modified diagnosis was the left parietal lobe tumor with ICH [
Figure 1:
In the computed tomography images (a): an ICH located in the parietal lobe surrounded by circle edema. An ellipse entity that had the same density as the cortex can be seen in the anterior part of this lesion. In MRI images: this lesion can be significantly enhanced in the contrast sequence (b), with an extremely low signal in the T1 sequence (c), and in the T2 (d) and FLAIR (e) sequence the signals were still low, which were different from the previous literature.
MANAGEMENT
Surgical procedure
This is a cortex-sub-cortex lesion that includes tumor and hematoma situated at the central region of the left hemisphere.
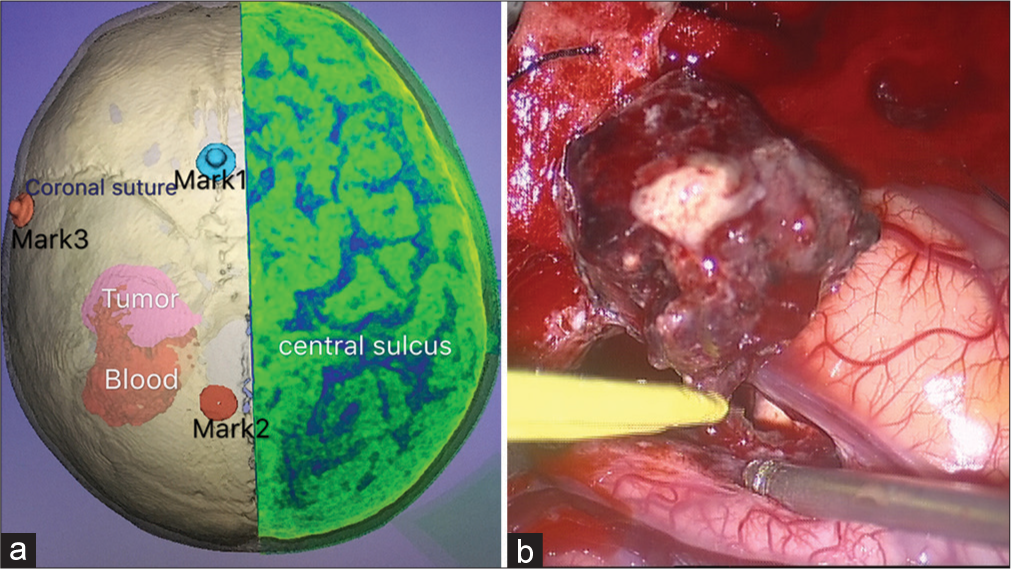
With the assistance of the neuronavigation, a left parietal craniotomy was performed. A trans-sulcus approach was used to perform the minimal cortical incision. We successfully removed all the hematoma and the gross total resection of the tumor was achieved [
Figure 2:
Three-dimensional reconstruction virtual image of the preoperative computed tomography scan showed the relationship between the tumor hematoma and the central sulcus [the left part of Figure 2]. The intro-operation image showed a gross total resection of the tumor, meanwhile, the cortical incision was limited [the right part of Figure 2].
Histological analysis
Gross specimen
The tumor is located in the superficial layer of the sub-cortex, with clear borders, 2 cm × 2 cm × 2.3 cm in size, gray-pink cut surface, and slightly tough texture.
Microscopical observation
The tumor is composed of glial components, blood vessels, and degenerated neurons. The tumor cells are spindle-shaped, bipolar, single-layered or multi-layered, arranged in a chrysanthemum-like structure, or forming a pseudo-chrysanthemum-like structure around the blood vessels, and ependymal-like structure; in some areas, spindle cells are interwoven into sheets to form a nerve sheath-like structure; tumor cell nuclei are oval, round, or spindle-shaped, with visible nucleoli and abundant cytoplasm. No nucleic mitosis was observed; the proliferation of microvessels, necrosis, and hemorrhage can be seen.
Immunohistochemical characteristics
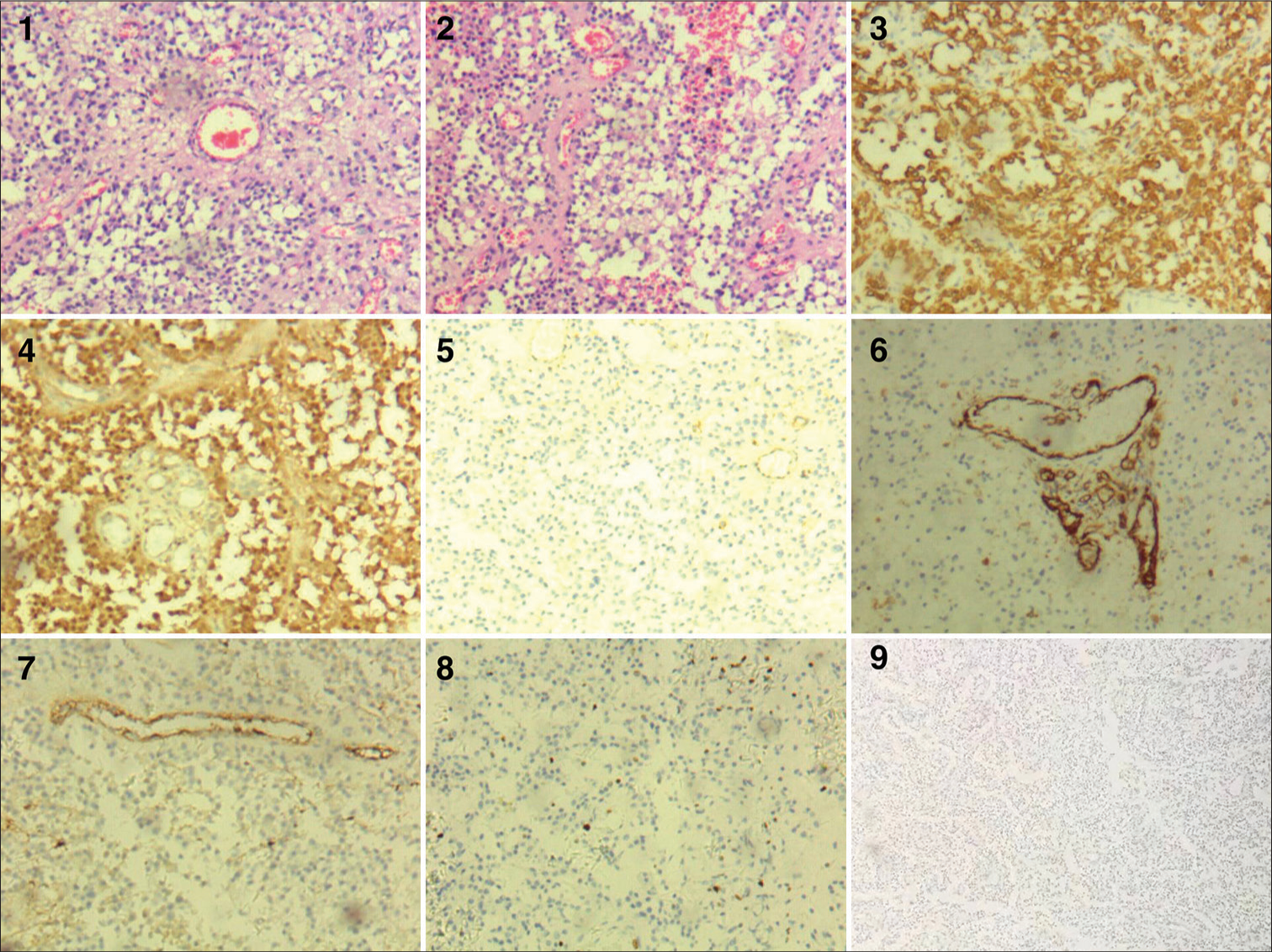
Tumor cells are diffusely positive for glial fibrillary acid protein (GFAP), S-100, CD99, blood vessels CD31, and F-8 are positive; proliferation index KI-67 hot spot is about 5%. Syn, CgA, NSE, AE1/AE3, and epithelial membrane antigen (EMA) are negative [
Figure 3:
(1 and 2) Hematoxylin and eosin staining. Spindle tumor cells, bipolar, single-layered or multi-layered, arranged in a chrysanthemum-like structure, or forming a pseudo-chrysanthemum-like structure around the blood vessels. Glial fibrillary acidic protein (3), S-100 (4), CD99 (5), CD31 (6), and F-8 (7) are positive. KI-67 hot spot is about 5% (8) EMA is negative (9). Original magnification ×100.
PROGNOSIS AND OUTCOMES
No postoperative complications occurred. After 2 weeks, the muscle strength of the right arm was reached level 3, and level 4 in her right leg.
Then, this patient was transferred to a rehabilitation center.
Due to its benign characteristic, adjuvant treatment such as chemo- or radiotherapy was not needed. Three months after discharge, the patient’s right limb hemiplegia was completely resolved.
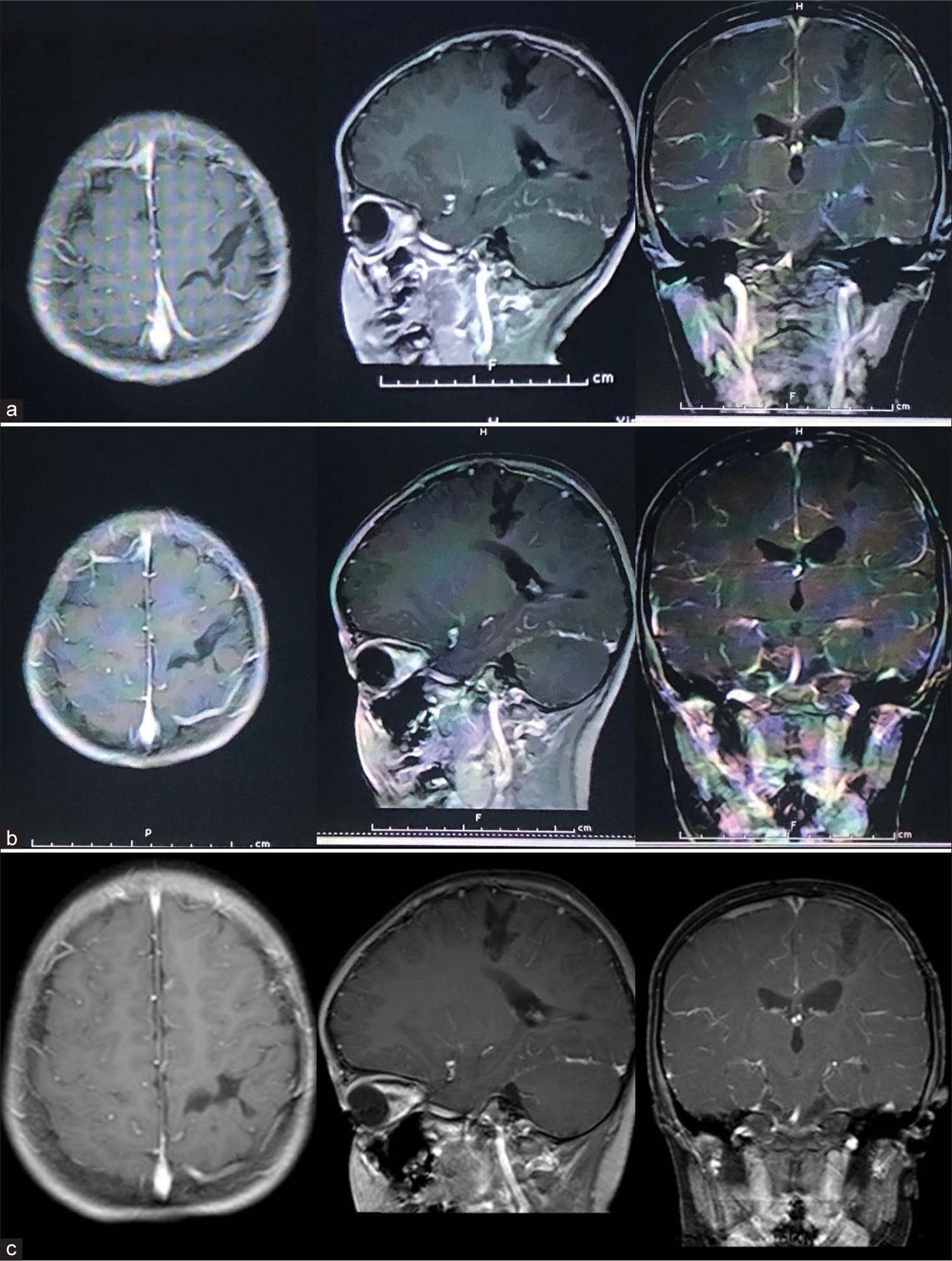
Twelve months later, there was no sign of tumor recurrence in the cranial MRI. After that, MRI examination was performed every 12 months, and the past MRI scan was 36 months later. No tumor recurrence was found till the past follow-up [
DISCUSSION
Here, we report a successfully surgically treated pediatric case of AG who presented with ICH and hemiplegia. The neurological condition of the child recovered fast. Long-term follow-up after 36 months showed no recurrence. AG was first reported by WANG and Lellouch-Tubiana in 2005 separately.
This is a rare brain tumor that mainly affects children and young people. Most patients have drug-resistance epilepsy as the chief complaint.[
In 2007, in the classification of tumors of the central nervous system, the WHO recognized it as a special type of central nervous system entity tumor for the 1st time.[
In 2016, it was classified as other gliomas. As a result of its benign biological behavior, the possibility of surgical resection, and low recurrence, it was attributed to the WHO Grade I.[
AG was considered an epilepsy-related neoplasm since it was first reported. In the previous literature reviews, most of the conclusions supported this argument.[
In a recent retrospect literature review, Han et al. summarized 108 cases, 90 patients (85.7%; 90/105) had a long history of various types of refractory seizures (the symptoms of three cases were not described). Only 15 (14.3%; 15/105) patients showed other symptoms.[
Our patient was the only case so far in the world literature who presented with an acute ICH, and never had a history of seizure no matter before or after the disease onset. This enriched AG’s clinical symptoms. A similar case was previously reported stating that a chronic hemorrhage inside the tumor with cystic changes may occur.[
The typical features on CT scan images have been previously described, in fact, there were no specificity on CT images, sometimes seemed such as edema, ischemic disease, or low-grade glioma. The diagnosis often was hard to be achieved only through the CT scan.[
In this case, we could observe hyper/iso-dense lesion located in the parietal lobe, anterior and superior to the hematoma. And circular low-density cerebral edema accompanied the lesion.
AG has similar features to low-grade gliomas. As previously described, the tumor’s characteristics on MRI are the hypointense signals in the T1 sequence and hyperintense signals in T2 and FLAIR sequence, without enhancement.[
The histopathological finding that we observed are in line with the findings described previously.[
Tumor cells were diffusely positive for GFAP, S-100, and CD99, but neuronal antigens such as CgA, NSE, and AE1/AE3 were negative. Proliferation index KI-67 hot spot is about 5%.
Blood vessels CD31, and F-8 were positive, necrosis, and hemorrhage, microvascular proliferation was observed.
Interestingly, EMA was negative which is different from the typical presentation. However, some cases in the three literature showed similar findings, reported by Arsene et al., Hu et al., and Sugita et al.[
Surgical procedure plays a fundamental role in the treatment of AG; gross total resection often achieved curative results, despite some cases showed a malignant process and tumor recrudescence.
After undergoing a successful operation, our patient did not receive any chemotherapy or radiotherapy. In most circumstances, adjuvant treatments are not necessary unless in cases with subtotal resection or biopsy.
We conducted a 36 months follow-up, the MRI scan performed every 12 months, till to now showing no signs of tumor recurrence.
CONCLUSION
Here, we present a case of AG, which has some unique characteristics, the onset of ICH without a history of epilepsy with some different MR images. Postoperative results and 36 months follow-up showed good tumor control. Microsurgery remains the main treatment option for AGs.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the hospital colleagues for their hard work and the cooperation from the patient’s family.
References
1. Adamek D, Siwek GP, Chrobak AA, Herman-Sucharska I, Kwiatkowski S, Morga R. Angiocentric glioma from a perspective of A-B-C classification of epilepsy associated tumors. Folia Neuropathol. 2016. 54: 40-9
2. Alexandru D, Haghighi B, Muhonen MG. The treatment of angiocentric glioma: Case report and literature review. Perm J. 2013. 17: e100-2
3. Arsene D, Ardeleanu C, Ogrezeanu I, Danaila L. Angiocentric glioma: Presentation of two cases with dissimilar histology. Clin Neuropathol. 2008. 27: 391-5
4. Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO classification of tumours of the central nervous system: Angiocentric glioma, pilomyxoid astrocytoma, and pituicytoma. Brain Pathol. 2007. 17: 319-24
5. Han G, Zhang J, Ma Y, Gui Q, Yin S. Clinical characteristics, treatment and prognosis of angiocentric glioma. Oncol Lett. 2020. 20: 1641-8
6. Hu XW, Zhang YH, Wang JJ, Jiang XF, Liu JM, Yang PF. Angiocentric glioma with rich blood supply. J Clin Neurosci. 2010. 17: 917-8
7. Koral K, Koral KM, Sklar F. Angiocentric glioma in a 4-year-old boy: Imaging characteristics and review of the literature. Clin Imaging. 2012. 36: 61-4
8. Lellouch-Tubiana A, Boddaert N, Bourgeois M, Fohlen M, Jouvet A, Delalande O. Angiocentric neuroepithelial tumor (ANET): A new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol. 2005. 15: 281-6
9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
10. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Sugita Y, Ono T, Ohshima K, Niino D, Ito M, Toda K. Brain surface spindle cell glioma in a patient with medically intractable partial epilepsy: A variant of monomorphous angiocentric glioma. Neuropathology. 2008. 28: 516-20
12. Wang M, Tihan T, Rojiani AM, Bodhireddy SR, Prayson RA, Iacuone JJ. Monomorphous angiocentric glioma: A distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 2005. 64: 875-81
13. Wang Q, Xiong Y, Chen J, Shao Q. Cystic angiocentric glioma: A case report and literature review. Childs Nerv Syst. 2020. 37: 2701-5