- Clinical Professor of Neurosurgery, School of Medicine, State University of NY at Stony Brook, and Editor-in-Chief Surgical Neurology International NY and ℅ Dr. Marc Agulnick 1122 Frankllin Avenue Suite 106, Garden City, NY 11530, USA,
- Assistant Clinical Professor of Orthopedics, NYU Langone Hospital, Long Island, NY, USA. 1122 Franklin Avenue Suite 106 Garden City, NY 11530.
Correspondence Address:
Nancy E. Epstein, M.D., F.A.C.S. Clinical Professor of Neurosurgery, School of Medicine, State University of NY at Stony Brook, and Editor-in-Chief Surgical Neurology International NY and ℅ Dr. Marc Agulnick 1122 Frankllin Avenue Suite 106, Garden City, NY 11530, USA.
DOI:10.25259/SNI_943_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein1, Marc A. Agulnick2. Perspective: Lumbar adhesive arachnoiditis (AA)/ Chronic AA (CAA) are clinical diagnoses that do not require radiographic confirmation. 04-Nov-2022;13:507
How to cite this URL: Nancy E. Epstein1, Marc A. Agulnick2. Perspective: Lumbar adhesive arachnoiditis (AA)/ Chronic AA (CAA) are clinical diagnoses that do not require radiographic confirmation. 04-Nov-2022;13:507. Available from: https://surgicalneurologyint.com/surgicalint-articles/11978/
Abstract
Background: Our hypothesis was that lumbar adhesive arachnoiditis (AA)/chronic lumbar AA (CAA) are clinical diagnoses that do not require radiographic confirmation. Therefore, patients with these syndromes do not necessarily have to demonstrate significant radiographic abnormalities on myelograms, MyeloCT studies, and/or MR examinations. When present, typical AA/CAA findings may include; central or peripheral nerve root/cauda equina thickening/clumping (i.e. latter empty sac sign), arachnoid cysts, soft tissue masses in the subarachnoid space, and/or failure of nerve roots to migrate ventrally on prone MR/Myelo-CT studies.
Methods : We reviewed 3 articles and 7 clinical series that involved a total of 253 patients with AA/CAA to determine whether there was a significant correlation between these clinical syndromes, and myelographic, Myelo-CT, and/or MR imaging pathology.
Results: We determined that patients with the clinical diagnoses of AA/CAA do not necessarily exhibit associated radiographic abnormalities. However, a subset of patients with AA/CAA may show the classical AA/CAA findings of; central or peripheral nerve root/cauda equina thickening/clumping (empty sac sign), arachnoid cysts, soft tissue masses in the subarachnoid space, and/or failure of nerve roots to migrate ventrally on prone MR/ Myelo-CT studies.
Conclusion: Patients with clinical diagnoses of AA/CAA do not necessary show associated neuroradiagnostic abnormalities on myelograms, Myelo-CT studies, or MR. Rather, the clinical syndromes of AA/CAA may exist alone without the requirement for radiolographic confirmation.
Keywords: Adhesive Arachnoiditis (AA), Chronic Adhesive Arachnoiditis (CAA), Clinical Syndrome, Diagnosis, Lumbar, Magnetic Resonance Imaging (MR), Myelo-CT Scans, Myelography, Mild-Moderate, Severe
INTRODUCTION
Patients with lumbar adhesive arachnoiditis (AA)/chronic AA (CAA) have clinical syndromes characterized by symptoms of pain, paresthesias, and varied motor, sensory, and/or sphincteric deficits. Our hypothesis was that patients with these syndromes do not have to demonstrate any significant radiographic confirmatory pathology on myelograms, Myelo-CT studies, or MR examinations.[
History of AA
In 1909, Sir Victor Horsely presented 21 cases where he anticipated finding spinal tumors. However, at surgery he encountered AA variously labeled as; chronic spinal meningitis/arachnoiditis, acute myelitis, adhesive spinal arachnoiditis, and meningitis serosa circumscripta spinalis [
Etiology of adhesive arachnoiditis (AA)/chronic adhesive arachnoiditis (CAA)
The major etiology of AA/CAA typically includes activation of a “…subarachnoid inflammatory cascade…” [
Typical clinical features of AA
Patients with AA may present with a wide array of neurological symptoms and signs [
MR and myelo-CT/plain film myelography (PFM) findings for AA/CAA
Myelograms, Myelo-CT studies, and MR examinations cited multiple imaging findings that may be associated with the clinical syndromes of AA/CAA [
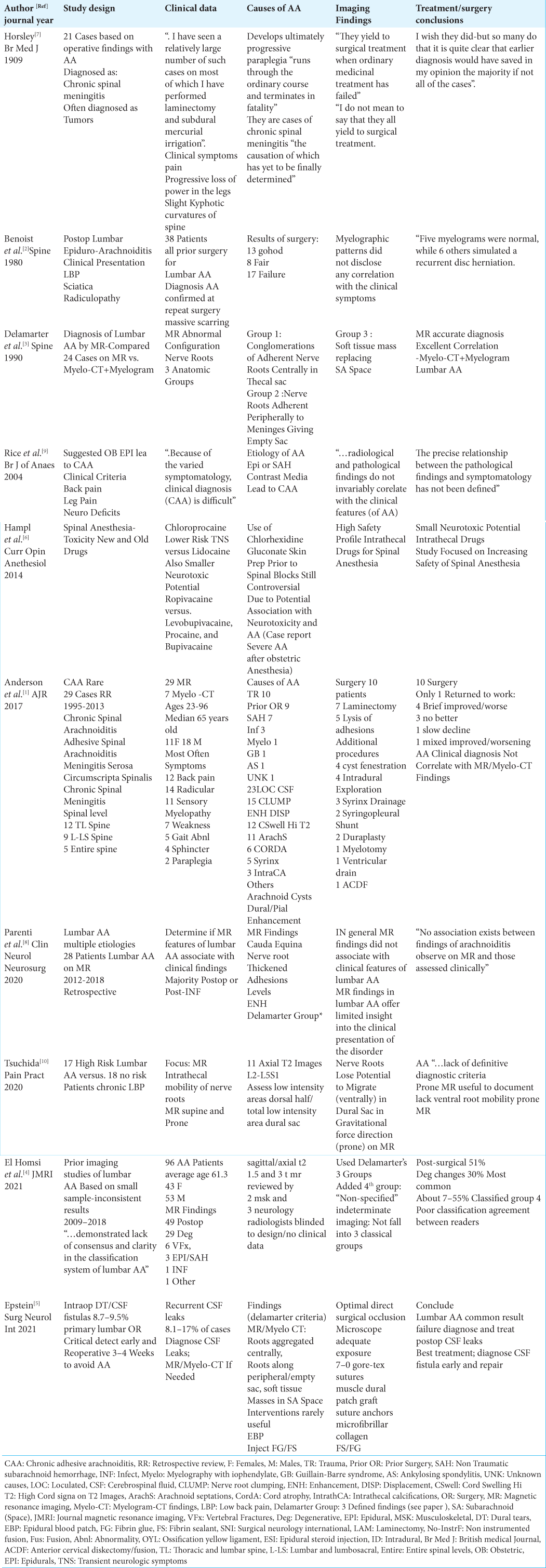
Benoist et al. findings [ Table 1 ][ 2 ]
In Benoist et al. (1980), 38 patients were diagnosed with lumbar AA attributed to prior lumbar surgery; all findings were confirmed at reoperations. Nevertheless, the authors found no specific correlation between the clinical syndrome of lumbar AA and any specific radiographic abnormalities; “Myelographic patterns did not disclose any correlation with the clinical symptoms…” [
Delamarter et al. findings [ Table 1 ][ 3 ]
In 1990, Delamarter et al., based on a review of 24 MR and 20 Myelo-CT/plain film myelographic (PFM) examinations, divided classical findings for AA into 3 Groups; Group I “… conglomeration of adherent nerve roots resting centrally within the thecal sac; Group II ”… nerve roots adherent peripherally to the meninges giving rise to an ‘empty sac’ appearance”; and Group III ”…showed a soft tissue mass replacing the subarachnoid space” [
Rice et al. (2004): Epidural anesthesia for delivery does not lead to CAA [ Table 1 ][ 9 ]
Rice et al. determined that patients who received epidural anesthesia for labor/delivery were not more susceptible to developing CAA as had been previously thought [
Anderson et al. findings [ Table 1 ][ 1 ]
Anderson et al. (2017) observed significant imaging findings (i.e. MR 29 patients: Myelo-CT 7 patients) in their 29 patients specifically selected for severe CAA [
Parenti et al. (2020) findings [ Table 1 ][ 8 ]
Parenti et al. (2020) noted variable MR findings for their 28 patients with AA; “…cauda equina nerve root clumping/ thickening, adhesion location/levels, enhancement, and (the) Delamarter group (criteria)” [
Tsuchida et al. findings (2020)[ 10 ]
Tsuchida et al. (2020) compared the MR scans performed in 17 “high-risk” AA vs. 18 “no risk” AA patients looking for failure of nerve roots/cauda equina to migrate ventrally on prone vs. supine studies [
El Homsi et al. findings (2021)[ 4 ]
In 2021, El Homsi et al. diagnosed 96 patients with lumbar AA using Delamarter’s 3 Groups [
Epstein (2021) findings [ Table 1 ][ 5 ]
Epstein (2021) reviewed Delamarter et al. classic 3 Groups based on MR and Myelo-CT studies for AA [
Lack of association between clinical and radiographic AA [ Table 1 ]
Multiple studies have shown that the clinical diagnoses of AA/ CAA are not necessarily corroborated by or associated with “diagnostic” radiographic AA abnormalities [
Surgery ineffective for lumbar AA [ Table 1 ]
Surgical intervention typically does not result in sustained neurological improvement for patients with AA/CAA [
CONCLUSION
The clinical diagnoses of AA/CAA do not require radiographic confirmation on myelographic, Myelo-CT, and/ or MR studies [
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Anderson TL, Morris JM, Wald JT, Kotsenas AL. Imaging appearance of advanced chronic adhesive arachnoiditis: A retrospective review. AJR Am J Roentgenol. 2017. 209: 648-55
2. Benoist M, Ficat C, Baraf P, Cauchoix J. Postoperative lumbar epiduro-arachnoiditis. Diagnostic and therapeutic aspects. Spine (Phila Pa 1976). 1980. 5: 432-6
3. Delamarter RB, Ross JS, Masaryk TJ, Modic MT, Bohlman HH. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990. 15: 304-10
4. El Homsi M, Gharzeddine K, Cuevas J, Arevalo-Perez J, Rebeiz K, Khoury NJ. MRI findings of arachnoiditis, revisited. Is classification possible?. J Magn Reson Imaging. 2021. 54: 904-9
5. Epstein NE. Perspective: Early diagnosis and treatment of postoperative recurrent cerebrospinal fluid fistulas/dural tears to avoid adhesive arachnoiditis. Surg Neurol Int. 2021. 12: 208
6. Hampl K, Steinfeldt T, Wulf H. Spinal anesthesia revisited: Toxicity of new and old drugs and compounds. Curr Opin Anesthesiol. 2014. 27: 549-55
7. Horsley V. A clinical lecture on chronic spinal meningitis: Its differential diagnosis and surgical treatment. Br Med J. 1909. 1: 513-7
8. Parenti V, Huda F, Richardson PK, Brown D, Aulakh M, Taheri MR. Lumbar arachnoiditis: Does imaging associate with clinical features?. Clin Neurol Neurosurg. 2020. 192: 105717
9. Rice I, Wee MY, Thomson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth. 2004. 92: 109-20
10. Tsuchida R, Sumitani M, Azuma K, Abe H, Hozumi J, Inoue R. A novel technique using magnetic resonance imaging in the supine and prone positions for diagnosing lumbar adhesive arachnoiditis: A preliminary study. Pain Pract. 2020. 20: 34-43