- Clinical Professor of Neurological Surgery, School of Medicine, State U. of NY at Stony Brook, NY and ℅ Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY 11530, USA.
Correspondence Address:
Nancy E. Epstein, M.D., Cllinical Professor of Neurological, School of Medicine, State University of New York at Stony Brook, NY, and ℅ Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY 11530, United States
DOI:10.25259/SNI_931_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E Epstein. Perspective on robotic spine surgery: Who’s doing the thinking?. 19-Oct-2021;12:520
How to cite this URL: Nancy E Epstein. Perspective on robotic spine surgery: Who’s doing the thinking?. 19-Oct-2021;12:520. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11192
Abstract
Background: Robotic assisted (RA) spine surgery was developed to reduce the morbidity for misplaced thoracolumbar (TL) pedicle screws (PS) resulting in neurovascular injuries, dural fistulas, and/or visceral/other injuries. RA is gaining the attention of spine surgeons to optimize the placement of TL PSs, and to do this more safely/effectively versus utilizing stereotactic navigation alone, or predominantly free hand (FH) techniques. However, little attention is being focused on whether a significant number of these TL RA instrumented fusions are necessary.
Methods: RA spine surgery has been developed to improve the safety, efficacy, and accuracy of minimally invasive TL versus open FH PS placement.
Results: Theoretical benefits of RA spine surgery include; enhanced accuracy of screw placement, fewer complications, less radiation exposure, smaller incisions, to minimize blood loss, reduce infection rates, shorten operative times, reduce postoperative recovery periods, and shorten lengths of stay. Cons of RA include; increased cost, increased morbidity with steep learning curves, robotic failures of registration, more soft tissue injuries, lateral skiving of drill guides, displacement of robotic arms impacting accurate PS placement, higher reoperation rates, and potential loss of accuracy with motion versus FH techniques. Notably, insufficient attention has been focused on the necessity for performing many of these TL PS instrumented fusions in the first place.
Conclusion: RA spinal surgery is still in its infancy, and comparison of RA versus FH techniques for TL PS placement demonstrates several potential pros, but also multiple cons. Further, more attention must be focused on whether many of these TL PS instrumented procedures are even warranted.
Keywords: Complications, Free hand: Pedicle screw placement, Morbidity, Robotic spine surgery: Neuronavigational, Skiving, Unnecessary fusions, Unwarranted surgery
INTRODUCTION
Robotic assisted (RA) spinal surgery was developed to reduce the frequency of neurological, vascular, visceral, and other injuries resulting from thoracolumbar (TL) pedicle screw (PS) fusions.[
HISTORY OF RA FOR SPINE SURGERY
In 2004, the FDA approved the first RA device for spinal surgery in the USA; Mazor Robotics Ltd. (Caesarea, Israel).[
COMPARISON OF ACCURACY OF RA VERSUS FH TL PEDICLE SCREW PLACEMENT
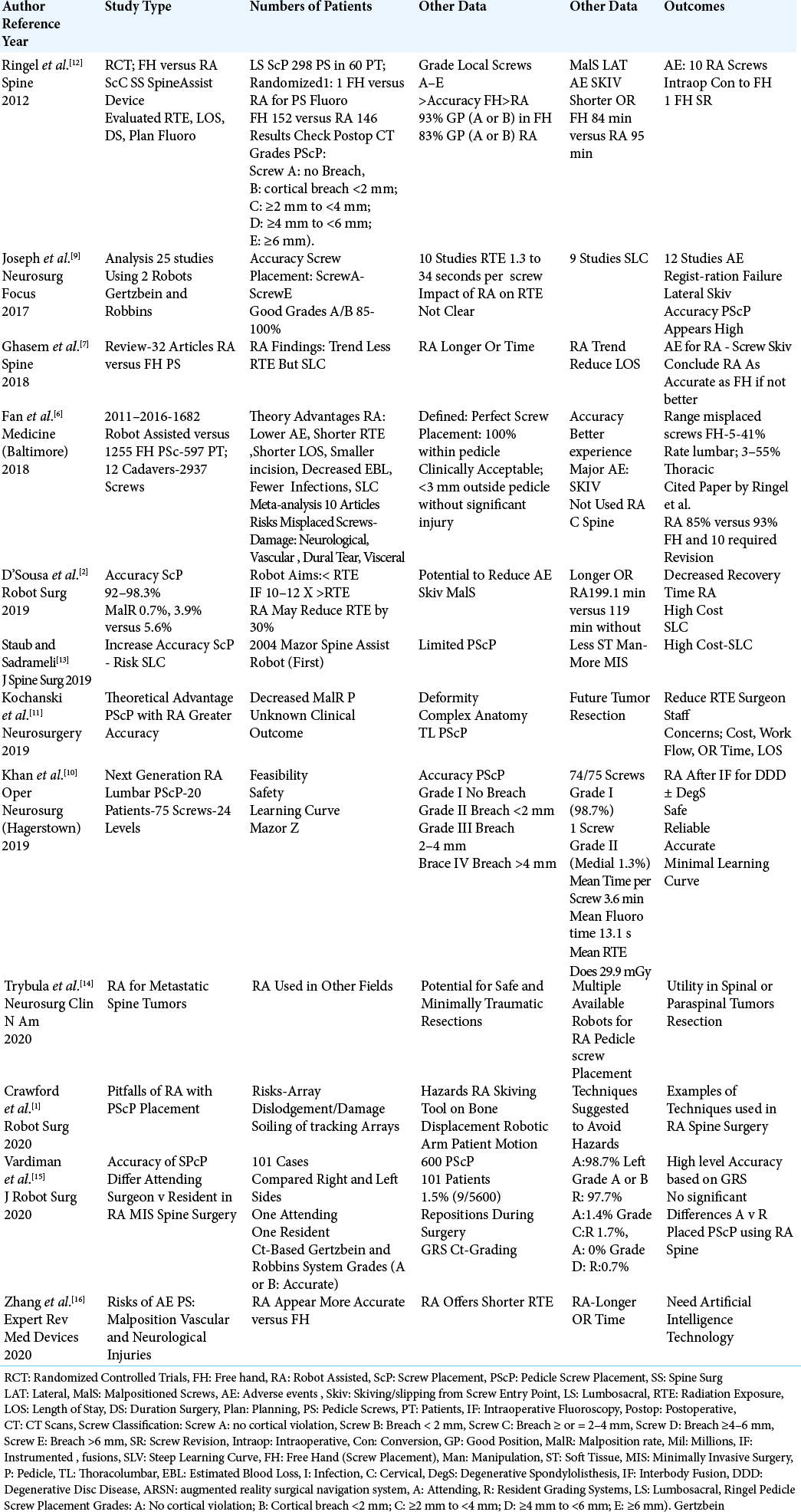
Different studies compared the safety/efficacy and pros/ cons of RA versus FH transpedicular screw placement in the thoracic or lumbar spine.[
SUPERIORITY OF FH OVER RA PLACEMENT OF PS IN THE LUMBAR SPINE
In a prospective randomized single center study, Ringel et al. (2012) looked at the safety/efficacy of PS placement for one or two-level lumbar fusions utilizing RA versus FH techniques [
SUPERIORITY OF RA VERSUS FH TECHNIQUES FOR TL PEDICLE SCREW PLACEMENT
Several studies documented the superiority of TL PS placement using RA-based versus FH techniques [
MINIMAL LEARNING CURVE AND PROS FOR RA VERSUS FH TL PS PLACEMENT
Minimal learning curve for RA versus FH screw placement
Two studies clearly documented a minimal learning curve for accurately placing TL PS using RA spinal techniqus [
Pros for RA versus TL PS Placement
Potential benefits for RA placement of TL PS included; greater safety/efficacy/accuracy of PS placement, reduced complications, the ability to adapt RA to intraoperative navigational techniques, less radiation exposure (i.e. to the patient, surgeon, and staff), shorter operative/postoperative recovery times, a shorter average length of stay, smaller incisions, reduced blood loss, and lower infection rates [
Steep learning curves and cons for RA spinal surgery
Cons for RA spinal surgery included; steep learning curves, greater cost, and technique-related difficulties compromising surgical accuracy [
TIMES FOR PREPARATION, SURGERY, AND RADIATION EXPOSURE WITH RA VERSUS FH
Comparable or shorter times for preparation, surgery, and radiation exposure using RA vs. FH techniques
When Ringel et al. (2012) evaluated 298 screws (FH 152, RA 146) placed in 60 patients, the preparation time, operating times, and intraoperative radiation exposure times were similar for both populations [
Longer times for preparation, surgery, and radiation exposure for RA vs. FH techniques
Several authors documented that RA spinal procedures required longer operative times, and higher doses/longer duration of radiation exposure versus FH techniques [
NEED TO RECONSIDER AND ACKNOWLEDGE HIGH RATE OF UNNECESSARY TL PS SPINE FUSIONS UTILIZING RA VERSUS FH TECHNIQUES
Future studies may better document that RA offers technological advantages over FH procedures for placing TL PS. Nevertheless, one should exercise better clinical judgment as to whether and when these procedures are warranted [
CONCLUSION
RA spinal procedures are still in their infancy as confirmed by the continued controversy regarding the relative safety/ efficacy and pros/cons of RA versus FH techniques for TL PS placement/instrumentation [
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
COMMENTARY 1
Training
The issue of what training is appropriate for RA surgery is an on-line course, a weekend course, incorporation of RA training within a spine fellowship, or the presence of the robotics representative for every case. Ideally, patients with significant polytrauma and multiple complex TL fractures will be treated in a tertiary care or trauma center. These tertiary centers are more likely to have a fellowship trained spine surgeon on staff, and a robot. This would allow for the volume needed to solve the problem of a steep learning curve, and enough familiarity with complex fractures, spinal biomechanics, and RA surgery to trouble shoot difficult cases such as highly unstable fractures which may move with patient positioning. The use of RA surgery could instill a false since of confidence in those surgeons who typically don’t do complex spine surgery to leave their comfort zone; this is concerning for the performance of inappropriate surgeries or unnecessarily long constructs.
Monitoring
Who monitors the accuracy of the robots-the surgeons, the robot representatives, or operating room personnel? Equipment that is moved for every case and potentially bumped multiple times is bound to lose accuracy over time. Accuracy should be checked between cases, not just when there is a miss or malfunction. From a medical-legal perspective, if the equipment is owned by the hospital, it would be their responsibility to ensure the equipment is checked unless a purchase agreement included a lifetime representative. It is also concerning in that any instrumentation can be used with a given robotic system. Or, on the other hand, does this force the surgeon to use the specific spinal instrumentation that is manufactured or distributed with the robot. This creates a situation of monopoly with the robot/instrumentation company, and risks having a surgeon use instrumentation that they are less familiar with which can increase the incidence of inadvertent mistakes, and additional OR time.
Costs
Does the potential accuracy of thoracic screw placement replace the need for intraoperative monitoring, or just add an additional cost of increased OR time and disposables? Does bundling of the robot and the spinal instrumentation increase the price of the spinal instrumentation in efforts to decrease the cost of the robot? Will RA be demanded by patients for all lumbar cases since it is a potential marketing tool? All these factors must be accounted for when determining the value and effiaccy in spinal surgery, otherwise we are driving costs up without added benefit.
Prof. Jamie Baisden MD
MCW-Neurosurgery
HUB 4th Floor
8701 Watertown Plank Rd
Milwaukee, WI 53226
Cell 262 902 0059
Phone: 414 248-4997
COMMENTARY 2
My impression before reading Nancy’s paper was that there was a substantial “learning curve” for the use of robotics and that Free Hand placement was very good in experienced surgeons hands.
How much time did the new technology take, was technical help necessary to run the equipment, did the equipment fail, was the final placement of the screws accurate, was there a higher infection rate because of the use of the technology, will the surgeon’s judgment be better because of the technology, or will his/her abilities be the same using the technology? In using navigation technology for brain surgery, I have found that the technology failed 1/3 of the time because of new people using it who were unfamiliar with it.
Her paper says just that. There is an impression being formed that robotic surgery will take over as a technology for spine surgery, which will make the manufacturers money. It is a new product from the manufacturer. They need to have new products. The learning curve is not important to them; that is the doctors’ problem, not theirs.
What you might want to consider is how many patients will be required to be successfully done using robotic technology to overcome the “learning curve”? Its’ complications should be counted as misplacement in the physician’s data and included in the reporting of results. For example, if a surgeon is doing a procedure and has 1 death, he/she needs to do 99 without any deaths to make the mortality rate 1%!
By “learning curve”, who plays the price? The patient. The reporting of complications can be questioned. For example, the interventionists only record complications for 24 hours! What about delayed rupture of an aneurysm, which I have seen, a week after the procedure, because of improper coiling. Isn’t that a complication? The “learning curve” complications in patients should be reported as complications, not as a “learning curve”.
How do people define complications? If the “learning curve” is used and discarded, or no complications are recorded after 24 hours, what does that mean compared to complication reporting as in the past. In previous times, all complications after the procedure, defined up to 30 days, were counted against the procedure or treatment. These complications would not have occured if the procedure was not done; so, they are all attributed to the choice of treatment.
I support new technology and ideas. But “compared to what?” is the question. There are ways to diminish the “learning curve” for new procedures. It is the physician’s responsibility to learn how to use the technology. This learning can be accomplished by practicing the new procedure in a laboratory, or morgue to diminish complications. Yes, that takes extra time and money, but that is what is best for the patient.
This is a different world. People are playing by different rules, their own rules to fit their agenda. That is not the actual TRUTH. But for some, truth does not matter. It is all about perception… and money.
James I. Ausman, MD. Ph.D.
Emeritus
Editor-In-Chief
Surgical Neurology International
References
1. Crawford N, Johnson N, Theodore N. Ensuring navigation integrity using robotics in spine surgery. J Robot Surg. 2020. 14: 177-83
2. D’Souza M, Gendreau J, Feng A, Kim LH, Ho AL, Veeravagu A. Robotic-assisted spine surgery: History, efficacy, cost, and future trends. Robot Surg. 2019. 6: 9-23
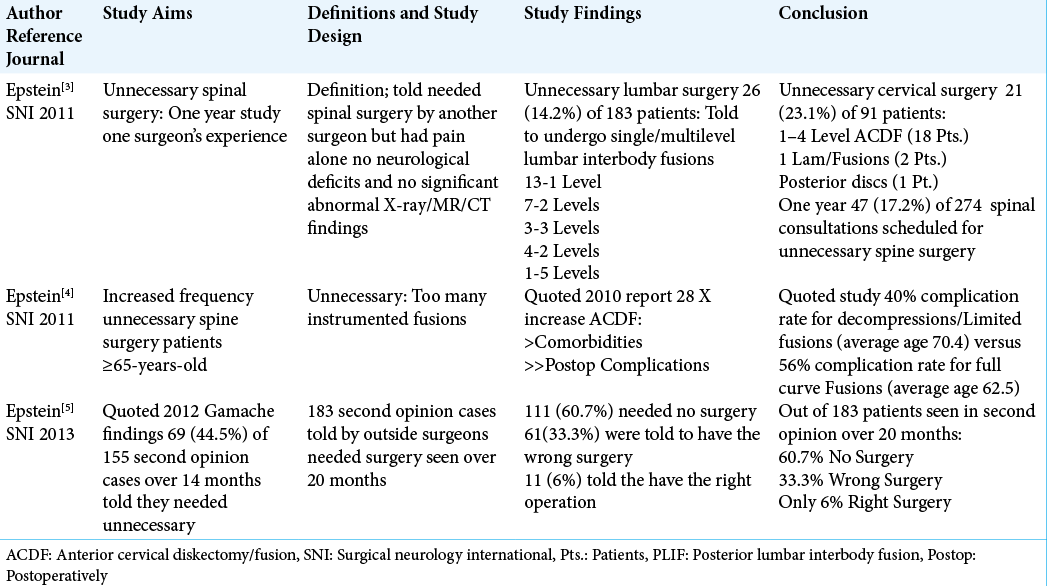
3. Epstein NE. “Unnecessary” spinal surgery: A prospective 1-year study of one surgeon’s experience. Surg Neurol Int. 2011. 2: 83
4. Epstein NE. Spine surgery in geriatric patients: Sometimes unnecessary, too much, or too little. Surg Neurol Int. 2011. 2: 188
5. Epstein NE. Are recommended spine operations either unnecessary or too complex? Evidence from second opinions. Surg Neurol Int. 2013. 4: S353-8
6. Fan Y, Du JP, Liu JJ, Zhang JN, Qiao HH, Liu SC. Accuracy of pedicle screw placement comparing robot-assisted technology and the free-hand with fluoroscopy-guided method in spine surgery: An updated meta-analysis. Medicine (Baltimore). 2018. 97: e10970
7. Ghasem A, Sharma A, Greif DN, Alam M, Maaieh MA. The arrival of robotics in spine surgery: A review of the literature. Spine (Phila Pa 1976). 2018. 43: 1670-7
8. Jiang B, Azad TD, Cottrill E, Zygourakis CC, Zhu AM, Crawford N. New spinal robotic technologies. Front Med. 2019. 13: 723-9
9. Joseph JR, Smith BW, Liu X, Park P. Current applications of robotics in spine surgery: A systematic review of the literature. Neurosurg Focus. 2017. 42: E2
10. Khan A, Meyers JE, Siasios I, Pollina J. Next-generation robotic spine surgery: First report on feasibility, safety, and learning curve. Oper Neurosurg (Hagerstown). 2019. 17: 61-9
11. Kochanski RB, Lombardi JM, Laratta JL, Lehman RA, O’Toole JE. Image-guided navigation and robotics in spine surgery. Neurosurgery. 2019. 84: 1179-89
12. Ringel F. Stuer C, Reinke A, Preuss A, Behr M, Auer F. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: A prospective randomized comparison to conventional freehand screw implantation. Spine (Phila Pa 1976). 2012. 37: E496-501
13. Staub BN, Sadrameli SS. The use of robotics in minimally invasive spine surgery. J Spine Surg. 2019. 5: S31-40
14. Trybula SJ, Oyon DE, Wolinsky JP. Robotic tissue manipulation and resection in spine surgery. Neurosurg Clin N Am. 2020. 31: 121-9
15. Vardiman AB, Wallace DJ, Booher GA, Crawford NR, Riggleman JR, Greeley SL. Does the accuracy of pedicle screw placement differ between the attending surgeon and resident in navigated robotic-assisted minimally invasive spine surgery?. J Robot Surg. 2020. 14: 567-72
16. Zhang Q, Han ZG, Xu YF, Fan MX, Zhao JW, Liu YJ. Robotic navigation during spine surgery. Expert Rev Med Devices. 2020. 17: 27-32