- Department of Neurosurgery, University of California – San Diego, San Diego, California, USA
Correspondence Address:
Reid Hoshide
Department of Neurosurgery, University of California – San Diego, San Diego, California, USA
DOI:10.4103/sni.sni_205_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Reid Hoshide, Justin Brown. Phrenic nerve decompression for the management of unilateral diaphragmatic paralysis – preoperative evaluation and operative technique. 24-Oct-2017;8:254
How to cite this URL: Reid Hoshide, Justin Brown. Phrenic nerve decompression for the management of unilateral diaphragmatic paralysis – preoperative evaluation and operative technique. 24-Oct-2017;8:254. Available from: http://surgicalneurologyint.com/surgicalint-articles/phrenic-nerve-decompression-for-the-management-of-unilateral-diaphragmatic-paralysis-preoperative-evaluation-and-operative-technique/
Abstract
Background:Unilateral diaphragmatic paralysis (UDP) can be a very disabling, typically causing shortness of breath and reduced exercise tolerance. We present a case of a surgical decompression of the phrenic nerve of a patient who presented with UDP, which occurred following cervical spine surgery.
Methods:The workup for the etiology of UDP demonstrated paradoxical movement on “sniff test” and notably impaired pulmonary function tests. Seven months following the onset of the UDP, he underwent a surgical decompression of the phrenic nerve at the level of the anterior scalene.
Results:He noted rapid symptomatic improvement following surgery and reversal of the above noted objective findings was documented. At his 4-year follow-up, he had complete resolution of his clinical symptoms. Repeated physiologic testing of his respiratory function had shown a complete reversal of his UDP.
Conclusions:Anatomical compression of the phrenic nerve by redundant neck vasculature should be considered in the differential diagnosis of UDP. Here we demonstrated the techniques in workup and surgical management, with both subjective and objective evidence of success.
Keywords: diaphragmatic paralysis, neurolysis, peripheral nerve surgery, phrenic nerve
INTRODUCTION
Unilateral diaphragmatic paralysis (UDP), secondary to phrenic nerve palsy, can be caused by a multitude of etiologies, but the actual cause is often elusive. It has been recently proposed that vascular compression from traversing vessels at the level of the thoracic outlet may contribute as in other compressive neurapraxias; in such a case surgical decompression may result in functional recovery.
Here we describe the preoperative assessment and the operative technique used to address a unilateral phrenic nerve palsy. Tightly adherent traversing vessels were identified at the time of exploration, specifically the transverse cervical and suprascapular arteries. Following ligation and sacrifice of these traversing vessels with neurolysis of the phrenic nerve over this segment, the patient's clinical symptoms resolved and the previously paralyzed diaphragm recovered.
PATIENT HISTORY
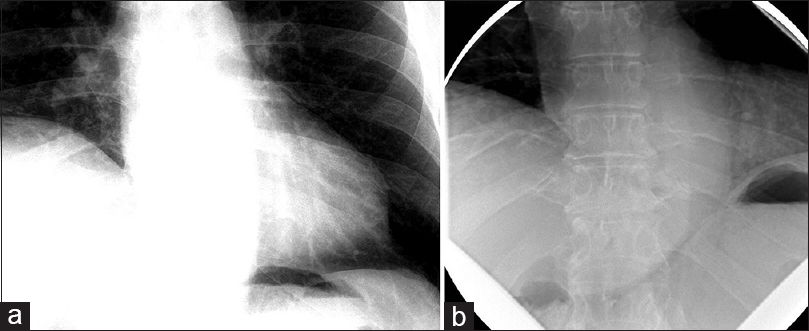
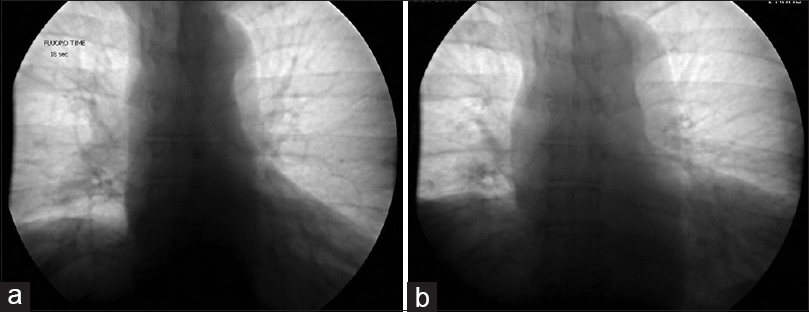
The patient was a 60-year-old male with a remote history of a motor vehicle accident who originally presented with symptoms of bilateral arm weakness and hyperesthesia, symptoms consistent with central cord syndrome. He underwent an anterior cervical discectomy and fusion at C5-C6 and C6-C7 levels through a left-sided approach. His weakness improved after surgery and he recalled no shortness of breath related to the accident or surgery at that time. Seven years later, he developed progressive myelopathic symptoms and was subsequently taken for a C4 to T2 laminectomy and posterior fusion, which was successful. Shortly following this operation, he began to experience shortness of breath. These symptoms persisted and gradually worsened over the course of 6 months to the point where he no longer had the respiratory stamina to do activities that he used to enjoy previously. At that time, the patient presented to our center and underwent workup for these symptoms. A fluoroscopic sniff test demonstrated paradoxical elevation of the right hemidiaphragm on rapid, forced inspiration with normal excursion of the left hemidiaphragm [
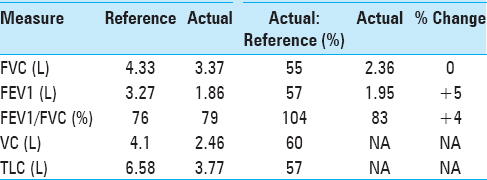
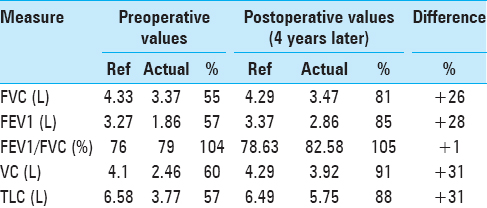
He additionally underwent pulmonary function tests (PFTs), which demonstrated findings similar to that of restrictive airway disease, as represented by a decreased 1-second forced expiratory volume (FEV1), decreased forced vital capacity (FVC), but with a preserved FEV1/FVC ratio. Moreover, he had a reduced total lung capacity (TLC) and vital capacity (VC). These values had negligible improvement following the administration of inhaled albuterol, distinguishing his diaphragmatic paralysis from intrinsic restrictive airway disease [
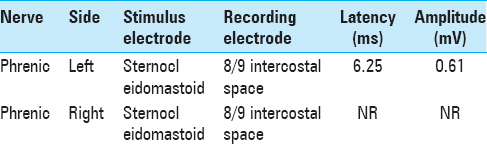
Analysis by electromyography (EMG) demonstrated an isolated, paralysis of the right phrenic nerve as evidenced by a lack of response at the recording lead within the diaphragm at the T8/9 intercostal space when stimulating the phrenic nerve at the level of the sternocleidomastoid [
It was concluded that his right hemidiaphragm was paralyzed due to a severe but incomplete right phrenic nerve injury. Due to the chronicity of this incomplete deficit, we offered a phrenic nerve exploration and decompression.
OPERATIVE TECHNIQUE
At 7 months from the onset of symptoms, the patient was taken for exploration of the phrenic nerve. He was brought to the operating room and placed under general anesthesia. The anesthesia team avoided the use of paralytic agents in order to preserve neuromonitoring capabilities during the case. The patient was positioned supine with his head turned to the left. A small bump was placed vertically between his shoulder blades to allow his shoulder to fall posteriorly, thereby maximizing the exposure of the right clavicular area. A 3 cm horizontal linear incision was made of a fingerbreadth superior to the clavicle, centered over the sternocleidomastoid. The platysma was divided, the sternocleidomastoid was retracted medially, and the supraclavicular fat pad was reflected superolaterally, exposing the anterior scalene. The phrenic nerve was identified on the surface of the anterior scalene with associated vessels crossing superficially, horizontally, and in contact with the phrenic nerve. These two arteries were running in parallel fashion immediately adjacent to one another – the suprascapular and transverse cervical arteries.[
In the immediate postop period, the patient did not endorse any improvement in his symptoms, nor did he describe any complications of the procedure. He was discharged home later that day after a period of postanesthesia observation.
Follow-up
At his 1-month follow-up, the patient reported that soon after surgery he developed a mild cramping sensation in his right chest wall at the level of the diaphragm, which was exacerbated by deep inspiration. In fact, he described that he would “guard” during deep inspiration due to the cramping pain. No testing was performed at this time.
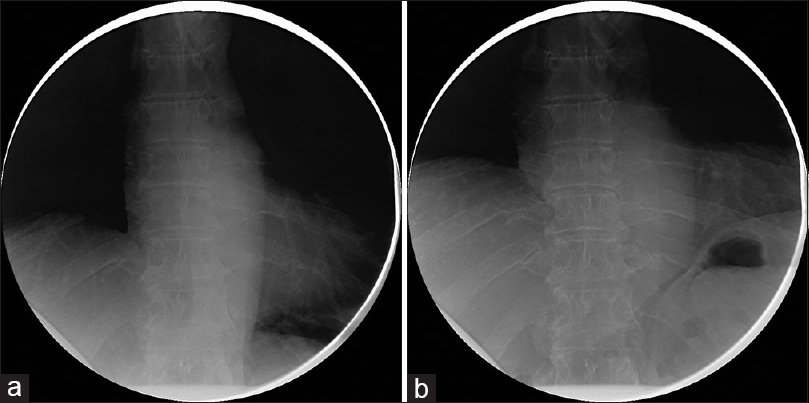
At his 6-month follow-up, he endorsed that the cramping pains had resolved shortly thereafter and he subjectively felt that his work of breathing improved. He noted that within a few weeks of the prior visit he felt that he was able to take fuller breaths than before. He was subsequently able to return to his normal level of activity, no longer finding himself “winded” when ascending his steep driveway. His incision was well-healed and he tolerated the sacrifice of the compressive arteries well. A sniff test was performed prior to this follow-up, revealing an improved excursion of the right diaphragm, now symmetric with the left diaphragm [Figure
Four years following surgery, he returned to our clinic after obtaining a new sniff test and a new PFT. At this time, the patient reported that he had returned to his baseline exercise tolerance. He had no additional neurological complaints. His PFTs performed at this visit demonstrated a significant improvement in FEV1, FVC, VC, and TLC [
His sniff test demonstrated durable improvement, and was stable compared to the previous sniff test performed 6 months postoperatively. The diaphragmatic excursion was symmetric in both the maximum exhalation and maximum inhalation sequences [Figure
Because of his overall improvement in objective measures (PFTs and fluoroscopic sniff test), coupled with his overall clinical subjective improvement, we decided not to pursue a new EMG/NCS study.
DISCUSSION
UDP is an uncommon affliction. The most common symptom of UDP is shortness of breath on exertion. However, many patients are asymptomatic and have incidental diagnoses based on chest radiographs for other reasons.[
Figure 4
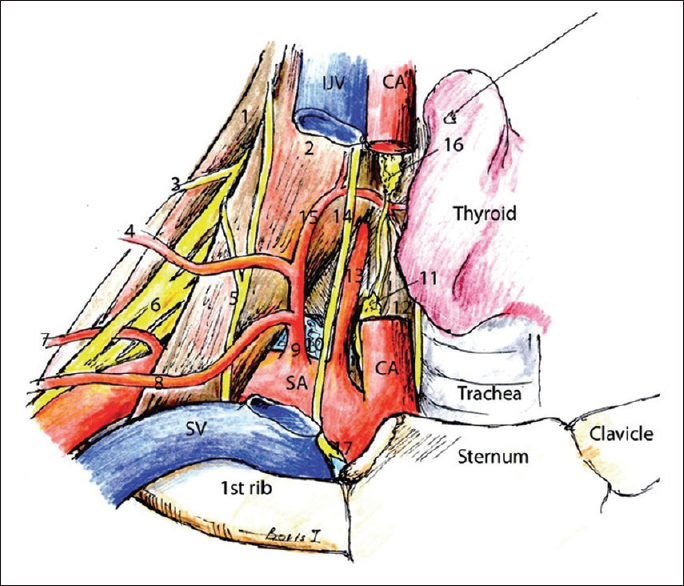
Illustration of the relationship between the arteries, musculature, and nerves of the clavicular area. (1) Middle scalene muscle, (2) anterior scalene muscle, (3) dorsal scapular nerve, (4) transverse cervical artery, (5) phrenic nerve, (6) brachial plexus, (7) dorsal scapular artery, (8) suprascapular artery, (9) thyrocervical artery, (10) lung, (11) inferior cervical sympathetic ganglion, (12) longus colli muscle, (13) vertebral artery, (14) vagus, (15) inferior thyroid artery, (16) middle cervical sympathetic ganglion, (17) recurrent laryngeal nerve. Original image reprinted from: Int J Shoulder Surg. 2010 Jul-Sep; 4 (3): 63–74. Creative Commons Attribution 4.0 (CC BY-SA 4.0). Full terms at
It was difficult to determine the exact pathophysiologic mechanism behind our patient's UDP. Certainly, patient positioning and shoulder traction in preparation for the surgery could have led to traction of the nerve, particularly given the anatomical arrangement at the level of the anterior scalene. Following anterior cervical discectomy and fusion, there is typically height added to the spine as the large intervertebral disc replacements are placed to distract the foramen. In doing so, the scalene muscles which originate from the transverse processes and insert on the first rib can experience additional traction. In a patient with a history of whiplash injury, the scalene muscles are frequently more fibrotic with thicker fascia and thus have less “give.” This could theoretically lead to tensioning of the scalene muscles and associated fascia and the invested structures (arteries), pressing them against adjacent nervous structures (the phrenic nerve in this case). Curiously, his symptoms arose immediately following a posterior cervical spine fusion, which does not typically result in the same type of cervical distraction. Ultimately, it could be inflammatory, such as a Parsonage-Turner Syndrome, or a combination of many different etiologies. In our case the exact cause of the phrenic palsy cannot be clearly identified and whether our intervention was the source of the improvement or simply corresponded temporally with the natural recovery of the nerve is impossible to determine.
The pathophysiologic mechanisms of shortness of breath in UDP are two-fold.[
Due to the rarity of this disease, there is no single well-studied course of management of patients with UDP. The single-most studied treatment has been diaphragmatic plication.[
Recent advances in surgical technique have aimed at reversing the etiologic causes of UDP. Kaufman et al. were the first to describe the concept presented here that ligation of the transverse cervical artery at the phrenic nerve may have contributed to restored diaphragmatic function immediately postoperatively in three patients who they believed suffered from a compressive vascular etiology.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Elefteriades J, Singh M, Tang P, Siegel MD, Kenney B, Pandey A. Unilateral diaphragm paralysis: Etiology, impact, and natural history. J Cardiovasc Surg (Torino). 2008. 49: 289-95
2. Fell SC. Surgical anatomy of the diaphragm and the phrenic nerve. Chest Surg Clin N Am. 1998. 8: 281-94
3. Graham DR, Kaplan D, Evans CC, Hind CR, Donnelly RJ. Diaphragmatic plication for unilateral diaphragmatic paralysis: A 10-year experience. Ann Thorac Surg. 1990. 49: 248-51
4. Higgs SM, Hussain A, Jackson M, Donnelly RJ, Berrisford RG. Long term results of diaphragmatic plication for unilateral diaphragm paralysis. Eur J Cardiothorac Surg. 2002. 21: 294-7
5. Kaufman MR, Willekes LJ, Elkwood AI, Rose MI, Patel TR, Ashinoff RL. Diaphragm paralysis caused by transverse cervical artery compression of the phrenic nerve: The Red Cross syndrome. Clin Neurol Neurosurg. 2012. 114: 502-5
6. Ko MA, Darling GE. Acquired paralysis of the diaphragm. Thorac Surg Clin. 2009. 19: 501-10
7. Krieger LM, Krieger AJ. The intercostal to phrenic nerve transfer: An effective means of reanimating the diaphragm in patients with high cervical spine injury. Plast Reconstr Surg. 2000. 105: 1255-61
8. McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med. 2012. 366: 932-42
9. Piehler JM, Pairolero PC, Gracey DR, Bernatz PE. Unexplained diaphragmatic paralysis: A harbinger of malignant disease?. J Thorac Cardiovasc Surg. 1982. 84: 861-4
10. Verenna AA, Alexandru D, Karimi A, Brown JM, Bove GM, Daly FJ. Dorsal scapular artery variations and relationship to the brachial plexus, and a related thoracic outlet syndrome case. J Brachial Plex Peripher Nerve Inj. 2016. 11: e21-8
11. Versteegh MI, Braun J, Voigt PG, Bosman DB, Stolk J, Rabe KF. Diaphragm plication in adult patients with diaphragm paralysis leads to long-term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg. 2007. 32: 449-56