- Department of Neurosurgery, Burdenko Neurosurgery Center, Moscow, Russian Federation.
- Department of Neuro-Ophthalmology, Burdenko Neurosurgery Center, Moscow, Russian Federation.
- Department of Neuropathology, Burdenko Neurosurgery Center, Moscow, Russian Federation.
Correspondence Address:
Dr. Irakliy Abramov, Department of Neurosurgery, Burdenko Neurosurgery Center, Moscow, Russian Federation.
DOI:10.25259/SNI_130_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alexander Konovalov1, David Pitskhelauri1, Natalia Serova2, Lyudmila Shishkina3, Irakliy Abramov1. Pineal cyst management: A single-institution experience spanning two decades. 12-Aug-2022;13:350
How to cite this URL: Alexander Konovalov1, David Pitskhelauri1, Natalia Serova2, Lyudmila Shishkina3, Irakliy Abramov1. Pineal cyst management: A single-institution experience spanning two decades. 12-Aug-2022;13:350. Available from: https://surgicalneurologyint.com/surgicalint-articles/11796/
Abstract
Background: Pineal cysts (PCs) are benign lesions commonly found on intracranial imaging. Despite their high prevalence, there is no clear consensus on the most appropriate management of patients with PCs, especially those with symptomatic nonhydrocephalic cysts.
Methods: A retrospective analysis was performed on 142 patients with PCs (103 surgical cases and 39 conservatively managed cases). Data were examined, including clinical presentation, imaging findings, ophthalmological status, natural course, postoperative outcomes, and complications.
Results: Surgical group: the most common symptom was headache (92%), followed by signs of intracranial hypertension due to hydrocephalus (22%). New radiological feature of PCs was found in 11 patients. From 71 patients with long-term follow-up, headache completely resolved in 44 (62%) patients; marked improvement was observed in 20 (29%); in 7 (9%) – headache remained unchanged. The most common postoperative complication was neuro-ophthalmological disorders (23%), with a tendency for resolution in the long-term follow-up period. Neuro-ophthalmological symptoms at last follow-up included upward gaze palsy (6%) and skew deviation (5%), followed by convergence disorders (3%) and eyelid-retraction (2%). Natural course group: PC size remained stable in 34 (87%) patients during the follow-up period. The patient’s gender or age was not a significant predictor of cyst growth (P = 0.4, P = 0.56).
Conclusion: The majority of patients with a newly diagnosed PC remain clinically and radiologically stable. Patients with nonhydrocephalic PCs and intractable headaches experience significant relief in headache symptoms, but are at risk of mild to moderate neuro-ophthalmological disorders. The natural course of PCs and factors promoting their growth still remains poorly defined.
Keywords: Headache, Hydrocephalus, Pineal cyst, Pineal region, Surgery
INTRODUCTION
Pineal cysts (PCs) are benign intracranial lesions that according to magnetic resonance imaging (MRI) studies account for 1.5–10.8% of the general population.[
Accurately defining the natural course of PCs has been challenging due to their predominantly asymptomatic course that results in incidentally discovered lesions which are consequently lost to follow-up. While hydrocephalus associated with PC represents the most common-accepted surgical indication, decision-making in nonhydrocephalic PCs is left to individual clinical judgment.[
This retrospective study reports the experience in management of patients harboring PCs at a single neurosurgical center for more than two decades. The purpose of this report is to describe the clinical manifestation and radiographic characteristics of symptomatic patients; to evaluate the surgical results and complications; to define the natural course of PCs that do not require surgical treatment.
MATERIALS AND METHODS
We retrospectively reviewed that all cases of PCs consecutively operated by two senior surgeons Dr Alexander Konovalov and Dr David Pitskhelauri at the Burdenko Neurosurgery Center between 1995 and 2018. Cases were identified using the medical and neuroimaging databases. Apart from surgical cases, we also studied the series of patients managed conservatively (the natural course group). The study was performed with the approval of the ethics committee at the Burdenko Neurosurgery Center. Informed consent for surgery was obtained from all patients.
Surgical group
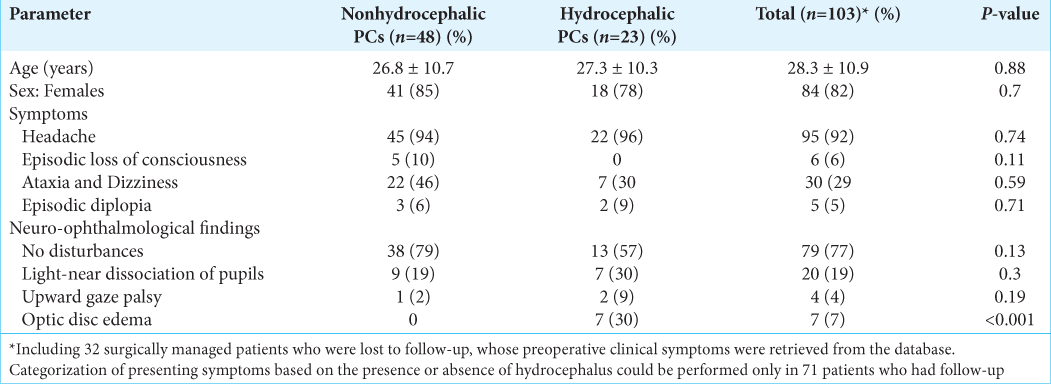
During this period, we operated on 103 symptomatic patients with PCs [
Our primary surgical approach for PCs resection is represented by the supracerebellar infratentorial approach in the sitting position (96% of our cases). The surgical route to the pineal region is opened after a midline linear skin incision, a small suboccipital craniotomy (3 × 4 cm), and a paramedian supracerebellar approach. When possible, PC resection is performed by its cautious detachment from the posterior and habenular commissures with their preservation, unless the tight adhesions of the cyst capsule are found.
Neuro-ophthalmological evaluation
Patients were categorized concerning the presence or absence of ophthalmological disturbances. Symptomatic patients were divided into mild and moderate groups depending on the severity of oculomotor and pupillary abnormalities. Patients with moderate ophthalmological disorders revealed on the preoperative examination were considered to be candidates for surgery. Mild disorders included: mild limitation of upward gaze, reduced pupillary light reflex, barely noticeable skew deviation, and no diplopia. Moderate disorders included: upward gaze palsy, eyelid-retraction, absence of pupillary light reflex, skew deviation, convergence-retraction nystagmus, and accommodation disorder. Subjective episodic diplopia alone was not considered as indication for surgery. We used our grading system for initial examination and after surgery in a short- and long-term follow-up period.
Headache assessment in patients without hydrocephalus
Particular caution was given during the initial workup of symptomatic patients with headaches and nonhydrocephalic cysts. None of these patients were offered surgical intervention on their first visit to the hospital. The headache’s severity and impact on the patient’s daily life were assessed by a team of neurologists and headache specialists. To determine headache impact on everyday life, Headache Impact Test was used.[
Radiological analysis
Available MRI sequences were studied and imaging characteristics were documented. To validate surgery in patients with nonhydrocephalic PCs suffering from intractable headaches, we used the previously described method of cerebral aqueduct (CAq) morphometrics assessment.[
Natural course group
The natural course group was composed of conservatively managed patients who had at least a 5-year clinical and radiological follow-up. Conservative management was focused on observation or symptomatic treatment with a specific emphasis on identifying the etiology of the headache. We opted for an observational strategy for patients with asymptomatic nonhydrocephalic PCs or clinical symptoms unrelated to the PC. Based on inclusion criteria, a list of 39 patients was generated. We reviewed initial clinical and radiological data for each patient and compared it with the last observation.
Statistical analysis
Statistical calculations were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). The continuous variables were presented as mean and standard deviation. Categorical variables were described using counts and percentages. Categorical variables in subgroups were explored using Chi-square test. Comparison between continuous variables was made using Mann–Whitney U-test.
RESULTS
Surgical group
Characteristics of the population
Neuro-ophthalmological examination findings were available in all cases preoperatively. In 79 (77%) patients, the preoperative ophthalmological examination showed no disturbances. Twenty (19%) patients were identified to have mild disorders resulting in light-near dissociation of pupils. Four (4%) patients presented with moderate disorders with upward gaze palsy. In 7 (7%) patients, optic disc edema due to hydrocephalus was documented, three progressed to optic atrophy.
Radiological features
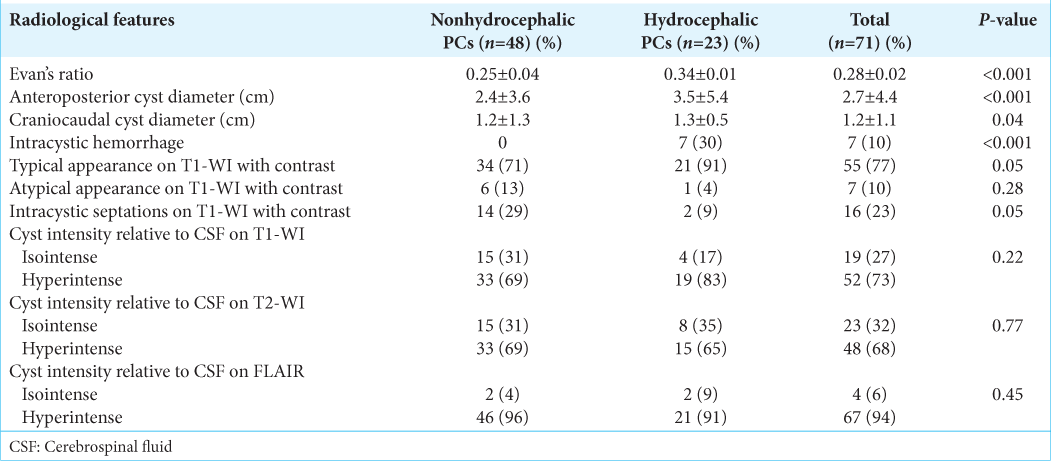
Detailed radiological data analysis was available for 71 patients [
The preoperative computed tomography (CT) scans were available in five cases, of which a thin rim of calcification along the cyst wall was observed in four cases.
In 77% of cases, on T1-weighted image, cysts were defined as typical ovoid lesions with smooth margins. It was noted that signaling intensity content was increased in 52 (73%) cases, while 19 (27%) patients’ signaling content was isointense relative to cerebrospinal fluid (CSF). On T1-weighted image enhanced with contrast, 55 (77%) cysts showed typical enhancement with a typical thin rim, while 16 (23%) had irregular margins with numerous intracystic septations and 7 (10%) showed nodular enhancement in the posterior aspect of the cyst. On T2-weighted image, 48 (68%) showed markedly increased signaling intensity and 23 (32%) were isointense relative to CSF. On FLAIR images, PCs appeared hyperintense relative to CSF in 67 (94%) cases and isointense in 4 (6%).
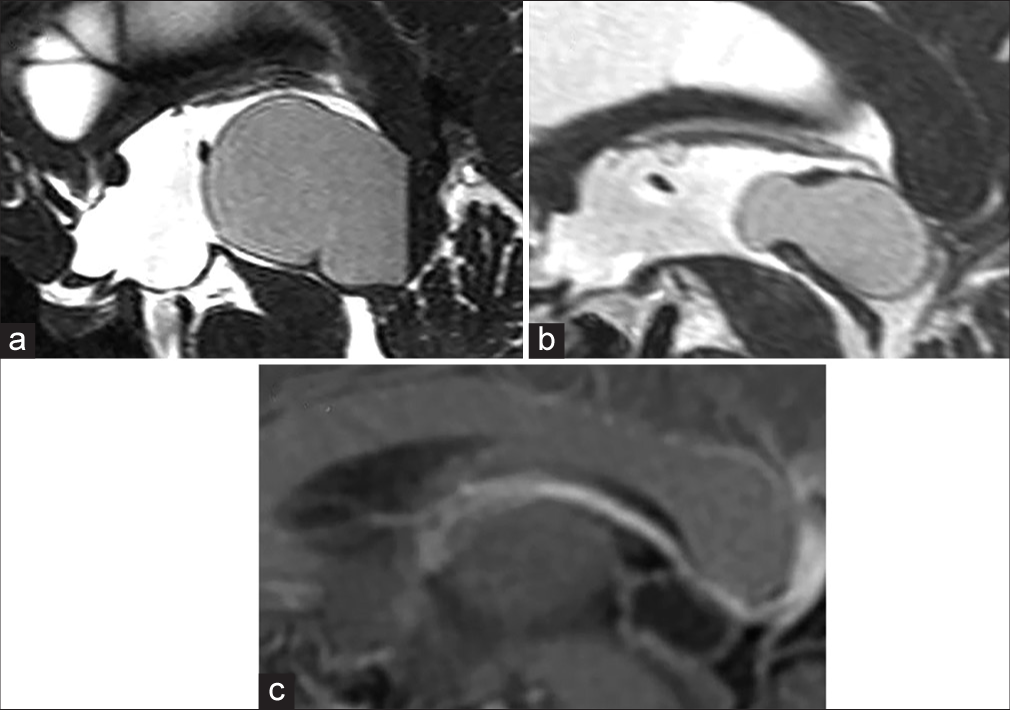
In 27 cases, sagittal FIESTA studies were available. According to this data, the cyst structure was classified into three types previously described by Pastel et al.:[
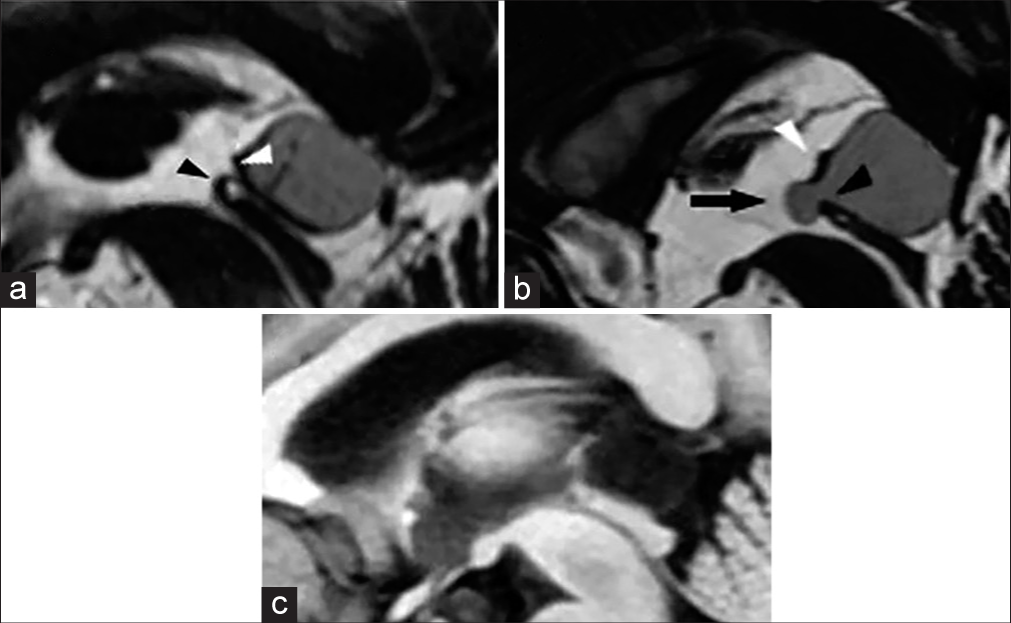
Figure 3:
(a) Midline sagittal FIESTA magnetic resonance imaging demonstrating a typical pineal cyst. (b) Midline sagittal FIESTA magnetic resonance imaging showing a pineal cyst with the diverticulum, which herniates between the habenular and posterior commissures. (c) The diverticulum of the same pineal cyst is not visible on the T1-weighted image with contrast-enhancement. Abbreviations: arrow, the diverticulum; white arrowhead, habenular commissure; black arrowhead, posterior commissure.
Surgery and outcome
Total resection of PC was achieved in all cases, which was confirmed by a postoperative MRI. In 99 (96%) patients, PC removal was performed in the sitting position through the supracerebellar infratentorial approach; 3 cases (3%) were operated through the anterior interhemispheric transcallosal approach; one (1%) patient underwent surgery through the occipital interhemispheric transtentorial approach. In 35 (35%) cases, when approaching the pineal region, 1–2 bridging veins were sacrificed. In 64 (65%), preservation of all bridging veins was achieved. While dissection of the quadrigeminal cistern, the precentral vein was preserved in 94 (91%) cases. In 9 (9%) cases, the precentral vein was coagulated and transected. Following resection of the PC, the posterior commissure was preserved in 63 (61%) cases and partially damaged in 4 (4%). The habenular commissure was preserved in 48 (47%) cases and disrupted in 16 (15%) [
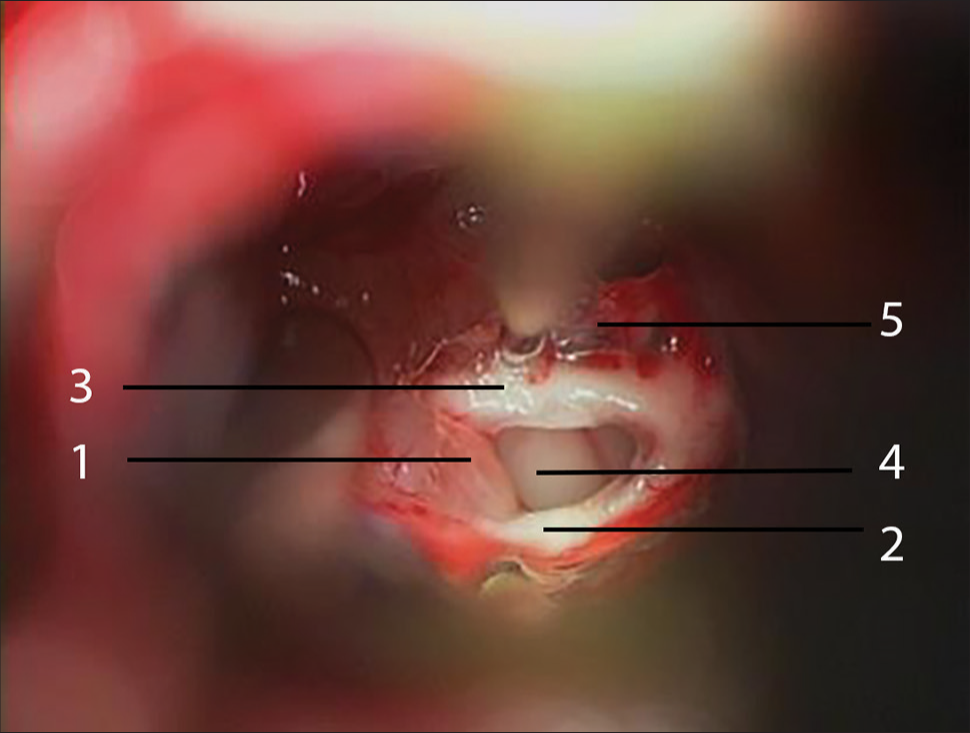
Figure 4:
Intraoperative illustration of the pineal cyst resection stage. After the pineal cyst (1) is emptied, its capsule is gently separated from the posterior (2) and habenular (3) commissures preserving their anatomical integrity. The third ventricle is exposed through the pineal (4) and suprapineal (5) recesses.
The mean discharge time after surgery was 8 ± 3 days. According to KPS scoring, 37 (36%) patients had a 100 score at discharge, 35 (34%) – 90 score, 29 (28%) – 80 score, 1 (1%) – 70 score, and 1 (1%) – 40 score.
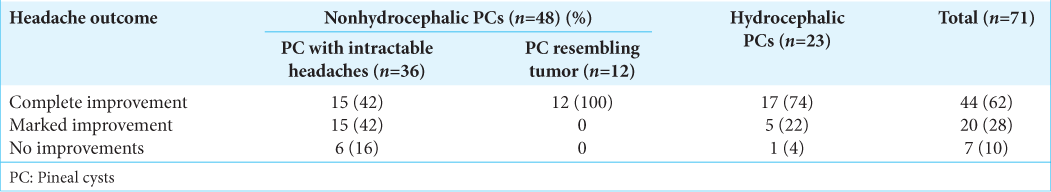
The clinical follow-up period was 5.4 ± 3 years (ranging from 6 months to 15 years). Of the 71 patients available for long-term follow-up, headache completely resolved in 44 (62%) patients. Reduction in headache severity and frequency, defined as marked improvement, was observed in 20 (28%). In 7 (10%) patients, headache remained unchanged [
Among 36 patients with nonhydrocephalic cysts who were operated on because of intractable headaches, 15 (42%) experienced total relief after surgery and 15 (42%) reported significant headache relief. In 6 (16%) patients, headache remained persistent. Patients with clinical improvement had a significantly lower preoperative Rd/Cd ratio (0.47 ± 0.15) when compared to patients with no improvements (0.75 ± 1.7) (P < 0.001).
Complications
There were no cases of postoperative mortality in our series. Thirty-seven (36%) out of 103 patients had some postoperative complications [
Neuro-ophthalmological outcome
Neuro-ophthalmology examination results were available in 79 patients for the short- and long-term outcome. These examinations were completed first on a postoperative day 1 or 2 and, then on average 8 ± 3 days after surgery, and 4 ± 3 years after surgery [
Figure 5:
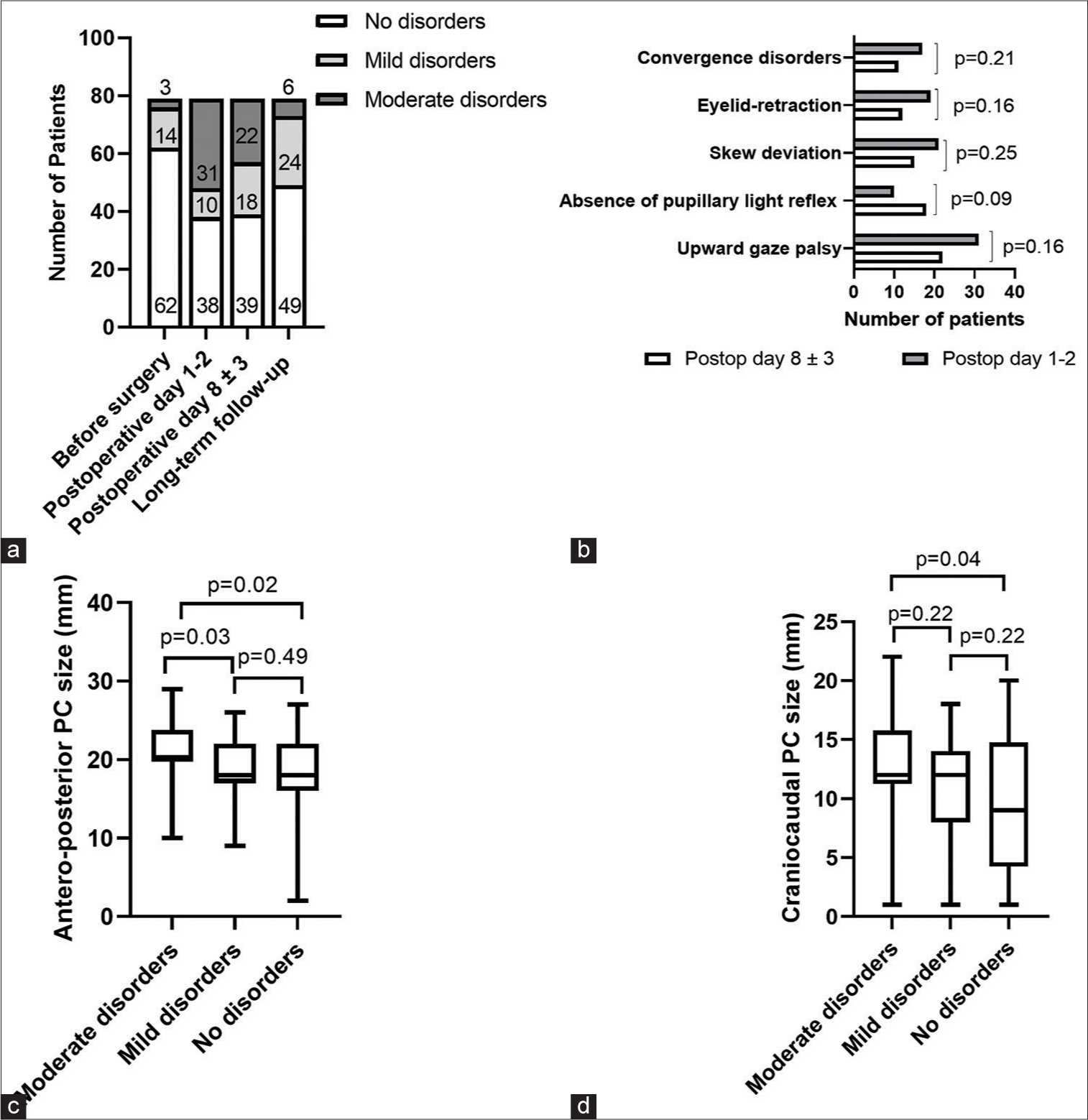
(a) The course of neuro-ophthalmological symptoms in the short- and long-term follow-up periods. (b) Detailed course of neuro-ophthalmological signs among patients with moderate disorders in the short-term postoperative period. Postop, postoperative. (c) Comparison of the antero-posterior PC size to different grades of neuroophthalmological disorders. Bars indicate standard deviations. (d) Comparison of the craniocaudal PC size to different grades of neuroophthalmological disorders. Bars indicate standard deviations.
On postoperative days 1–2, we observed a tendency for a significant proportion of patients to decline in their neuroophthalmological status (P < 0.001). Of 62 (79%) patients presenting without initial neuro-ophthalmological disorders, 38 (48%) retained normal pupillary reflex and ocular motility on examination on a postoperative day 1–2; 41 patients deteriorated and had mild (13%) and moderate (39%) neuroophthalmological disorders. Transient moderate neuroophthalmological disorders in the short-term postoperative period were seen in 9 (11%) patients. We did not observe significant improvements in the neuro-ophthalmological status of patients by the postoperative day 8 ± 3, with a total of moderate disorders seen in 22 (27%) patients. The detailed course of neuro-ophthalmological deficits in patients with moderate disorders in the short-term period is represented in
Analysis of neuro-ophthalmological symptoms in the long-term follow-up period showed that most patients had mild (30%) or no deficit (62%), when compared to the short-term outcomes. Of 31 patients with moderate disorders on postoperative days 1–2, symptom resolution was observed in 82% in the long-term follow-up period. Overall, among patients with no neuro-ophthalmological symptoms before surgery, 79% were asymptomatic by the end of the long-term follow-up. The most common symptoms at last follow-up were upward gaze palsy (6%) and skew deviation (5%), followed by convergence disorders (3%) and eyelid-retraction (2%). There were no patients with moderate disorders who did not improve by 5 years after surgery.
Intraoperative injury of the posterior commissure was not associated with the presence of moderate neuroophthalmological disorders (P = 0.26). However, we found that patients with moderate neuro-ophthalmological disorders were associated with significantly greater anteroposterior cyst diameter, when compared to patients with mild disorders (21 ± 3.9 vs. 18.8 ±3.6, P = 0.03) and no disorders (21 ± 3.9 vs. 18 ± 5.2, P = 0.02) [
Natural course group
The male/female ratio was 1:3 (9 male, 30 female). The mean age at diagnosis was 35 ± 13.5 (ranging from 5 to 62 years). The most common presenting symptom was headache (63%, n = 25). Other symptoms included dizziness (n = 6), nausea and vomiting (n = 6), numbness of fingertips (n = 3), tinnitus (n = 2), and memory disorders (n = 2). In all cases, after initial medical PCs were judged to be an incidental finding unrelated to the presenting symptoms. Five patients had reduced pupillary light response. None of the patients presented with moderate neuro-ophthalmological disorders or hydrocephalus. The mean anteroposterior cyst diameter was 1.5 ± 0.4 cm (ranging 0.7–2.4) and craniocaudal diameter – 0.9 ± 0.3 cm (ranging 0.4–1.4). PC size in the natural course group was significantly smaller than in the surgical group (P = 0.001).
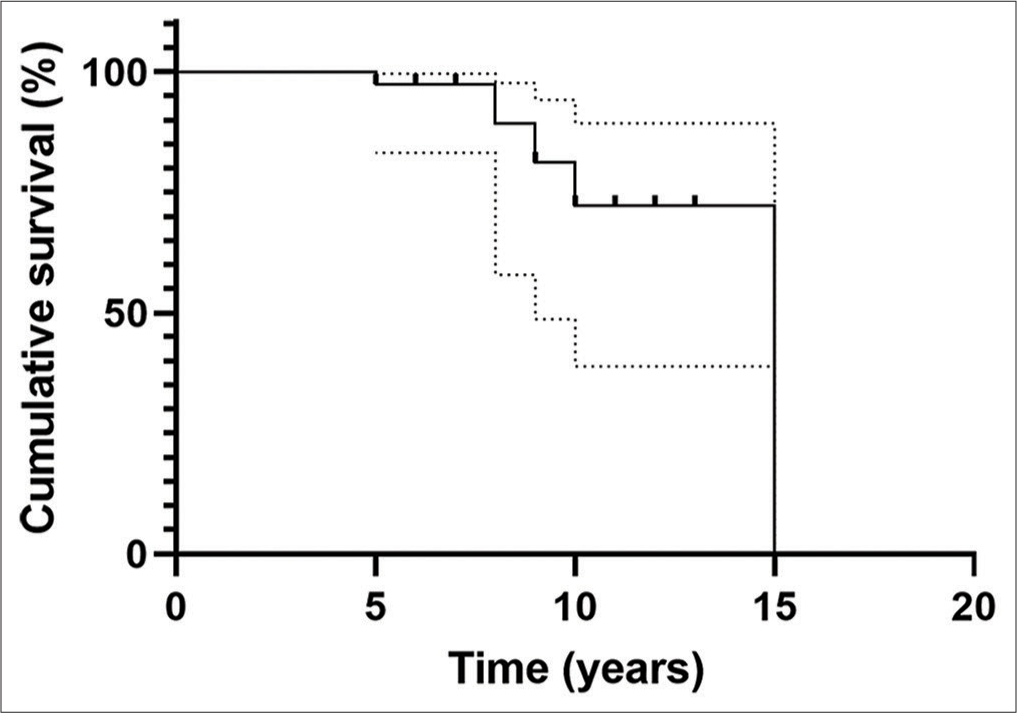
The follow-up interval varied from 5 to 15 years, with the mean interval being 7 ± 2.5 years. Headache resolved in 14 (56%) of patients and improved in 11 (44%). Other nonspecific symptoms improved significantly or resolved in all patients. PC enhancement and signaling intensity characteristics did not change in the 39 patients on the last follow-up MRI. PC size remained stable during the follow-up in 34 (87%) out of 39 patients [
DISCUSSION
Clinical and radiological features
PC is one of the most common brain lesions diagnosed in predominantly young females and yet accompanied with nonspecific symptoms most of the time. Despite the high prevalence, identification of symptomatic cysts remains an unsolved problem.
Discrepancies in the incidence of preoperative neuroophthalmological symptoms occur throughout the published studies on surgically managed PCs, ranging between 2% and 72.5%.[
The previous studies deemed large cysts to have the potential to cause symptoms.[
PCs can have an atypical nodular enhancement in 11% of cases.[
In addition, our analysis of FIESTA sequences discovered a new type of PC, which has never been reported previously in the literature. A feature of this type of PC is that the anterior portion of the cyst herniates in the third ventricle above the CAq. We hypothesize that the herniating part can act as a ball valve, moving in the anterior-inferior direction with subsequent aqueductal stenosis and intermittent obstruction of the CSF flow. It is important to note that the herniating part is barely visible on the common MRI sequences. Given that herniating part may act as a ball valve, patients with this type of PC might experience symptoms related to the intermittent obstruction of CSF. In our cohort, this irregularly-shaped cyst was observed in 11 patients, all of whom presented with severe headaches (n = 10), episodic loss of consciousness (n = 1), and dizziness (n = 3), which resolved immediately after surgery. Therefore, FIESTA sequence is a valuable tool for PC management. The current observation of herniated PCs can aid in further investigations to substantiate the hypothesis.
Surgery and complications
The literature and acceptance for consideration of surgery in patients with hydrocephalus or Parinaud’s syndrome are not in question. The surgical approach to a PC that is large enough to cause hydrocephalus could be dealt with endoscopically or with the use of a microscope. In many cases, endoscopic strategies may include cyst fenestration with or without an endoscopic third ventriculostomy without cyst resection.[
In our center, microsurgical techniques and the supracerebellar infratentorial approach in the sitting position are most commonly utilized for PC resection (96%). The most significant disadvantage and why many neurosurgical centers avoid the sitting position for the supracerebellar approach is venous air embolism-associated complications. In a systematic review comparing craniotomy in the sitting and supine positions, the incidence of venous air embolism was 15–45% versus 0–12%, respectively.[
In three cases, the PC resembled a tumor causing hydrocephalus, and thus, an anterior interhemispheric transcallosal approach with a simultaneous microsurgical third ventriculostomy was preferred.[
The preservation of bridging veins and the precentral vein was not possible in 35% and 9% of the cases, respectively. Despite this fact, we did not observe any neurological deterioration or cerebellar edema in these patients following surgery.
Neuro-ophthalmological outcome
Since one of the most common post-operative complications in our study was associated with moderate neuro-ophthalmological disorders, we precisely evaluated its course in the short- and long-term postoperative periods. Available studies regarding neuro-ophthalmological outcomes after PC surgery are scarce and do not describe the course of symptoms in the postoperative period.[
We found that long-term neuro-ophthalmological disturbances were more common and long-lasting than expected. Among patients with no or mild disorders before surgery, 34 (38%) had a marked increase in oculomotor and pupillary disorders on postoperative day 1–2. However, acquired disturbances showed a tendency to partially regress by the time of discharge – on review on postoperative day 8 ± 3. Noteworthy, the most common symptoms at last follow-up were upward gaze palsy (6%) and skew deviation (5%).
We found that moderate disorders were significantly related to the cyst size. Hence, the incidence of postoperative neuroophthalmological deficits reported in our study probably reflects the tense adhesion of relatively large PCs to the posterior commissure. Furthermore, surgeon’s diligence to a total resection and the number of manipulations that he performs while detaching the cyst from the posterior commissure may also play an important role in such disorders. The rostral intermediate medial longitudinal fasciculus decussates in the posterior commissure, conveys neuronal interconnections with the interstitial nucleus of Cajal, and is associated with the upward gaze control.[
Headache outcome in non-hydrocephalic PCs
Patients suffering from intractable headaches have a lower quality of life.[
Our previous study, which was conducted in 2016, hypothesized that PC-related CAq stenosis might cause intractable headaches in the absence of hydrocephalus. We described the method for CAq morphometrics measurement and investigated the clinical outcome concerning different aqueduct geometry.[
Natural course group
We found that the majority of patients with PCs remain clinically stable during follow-up, with a complete resolution or improvement in their symptoms. According to our analysis, patients with a newly diagnosed PC remain radiologically stable at least 5 years after diagnosis [
It is important to note that in one patient hydrocephalus developed after 8 years of follow-up. This observation is an important finding regarding the aspects of PC’s natural course. Initially, asymptomatic PC can grow over the years and cause hydrocephalus.
Management algorithm
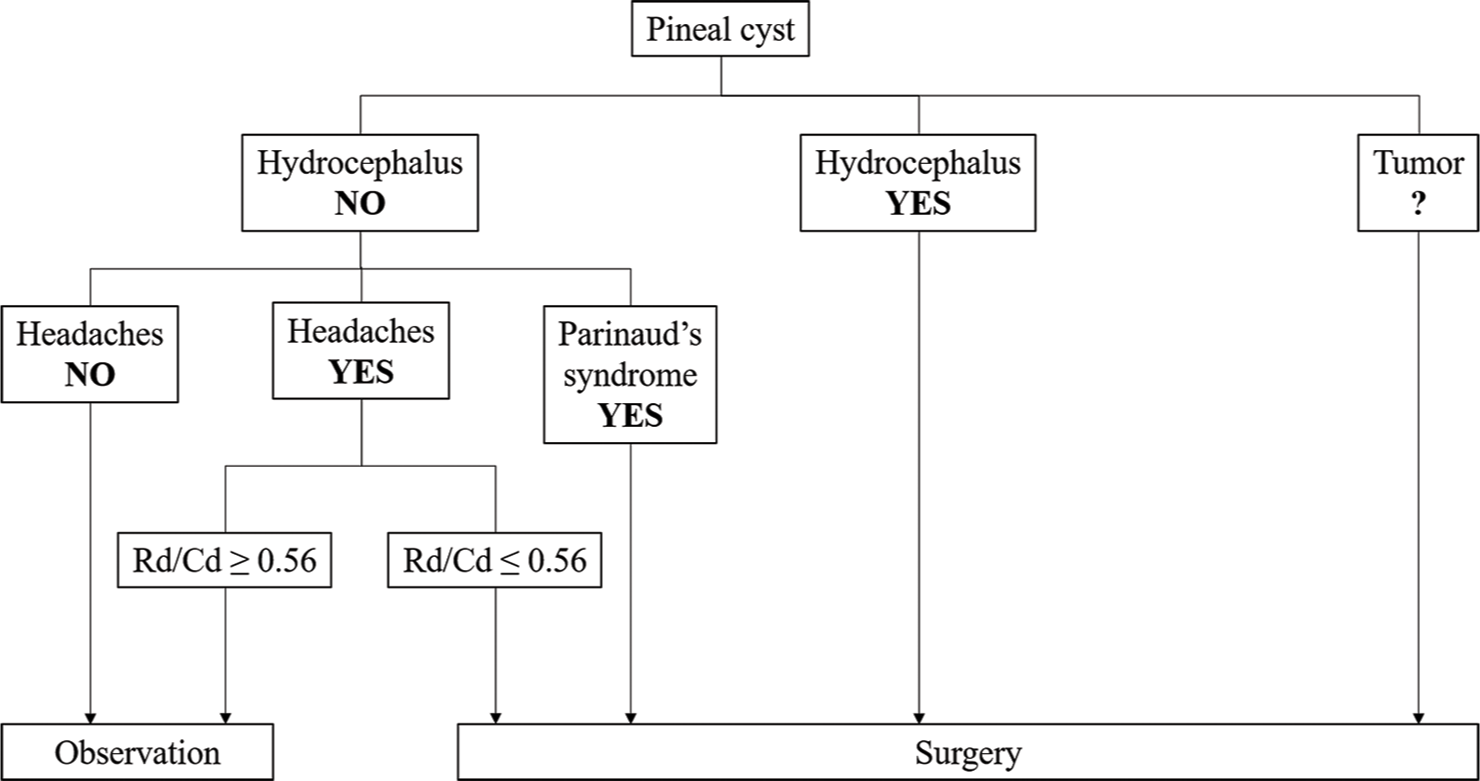
We have developed a management algorithm based on the patterns and distinguishing features of PCs obtained in the present study [
Patients with atypical MR characteristics of PC, such as atypical contrast-enhancement, irregular cyst shape, a predominance of the solid component over cystic, require first of all, differential diagnosis with lesions of the pineal region. Decision-making in the management of such patients can be addressed through a close follow-up with repeat imaging in 6 months and assessment of tumor markers (alpha-fetoprotein and beta-chorionic gonadotropin). In the cases of radiologically unstable atypical PC (cyst growth or change in radiological patterns) and negative tumor markers, surgical intervention could be considered.
In cases when a patient with nonhydrocephalic PC suffers from headaches, determining the cause of this symptom is paramount. A thorough neurological examination and other specialists aimed at excluding conditions associated with the primary and secondary nature of the headache are fundamental.
In addition, the algorithm addresses the preoperative Rd and Rd/Cd ratio, the two crucial factors in the management of patients with headache and nonhydrocephalic cysts. The first line of management in such patients should be an observational strategy with conservative therapy over a 2–3 month period of time. Resistance to medication during a given period, absence of any conditions associated with headache, and preoperative Rd/Cd ratio of the CAq ≤0.56 is positive indicators for surgical intervention.
Limitations
Several limitations should be acknowledged. The present study has a retrospective nature with an insufficient level of evidence and associated selection biases. Thirty-two surgically treated patients could not be recalled to assess their radiological features and outcome, which introduces the loss to follow-up bias. The prevailing reasons for the loss to follow-up occurred due to the migration of the patients or their “lack of time” to undergo follow-up evaluation (29 patients). In addition, some patients were operated in early 2000s, before the follow-up protocol was established in our center. The dropout rates could severely compromise the study’s validity if reluctance to undergo follow-up screening is associated with mistrust due to poor clinical outcomes. Regarding the three patients with a loss to follow-up due to death, the association with surgery is less likely, since all three had KPS 100 at discharge. In addition, there is a selection bias for the natural course group, since only patients without indications for surgery were included in the group. The true natural history of PCs would be determined by a random inclusion of patients with diagnosed PC and subsequent prospective follow-up. Finally, the present population of surgically treated patients overlaps for 30 patients with our previous study on the analysis of CAq diameter. Nevertheless, we believe that this study highlights essential data on developing the right approach to a patient with a newly diagnosed PC.
CONCLUSION
In this study, we attempted to answer a series of questions regarding the symptomology, radiological features, natural course, postoperative follow-up, and complications in patients with PC. Patients with nonhydrocephalic PCs and intractable headaches experience significant relief in headache symptoms, but are at risk of mild-to-moderate neuro-ophthalmological disorders. Still, many aspects of PC’s natural course remain unsolved. Further research should focus on prospective clinical trials combined with novel imaging tools.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abramov IT, Pitskhelauri DI, Serova NK. Pineal cyst. Zh Vopr Neirokhir Im N N Burdenko. 2017. 81: 113-20
2. Al-Holou WN, Garton HJ, Muraszko KM, Ibrahim M, Maher CO. Prevalence of pineal cysts in children and young adults. Clinical article. J Neurosurg Pediatr. 2009. 4: 230-6
3. Al-Holou WN, Terman SW, Kilburg C, Garton HJ, Muraszko KM, Chandler WF. Prevalence and natural history of pineal cysts in adults. J Neurosurg. 2011. 115: 1106-14
4. Barboriak DP, Lee L, Provenzale JM. Serial MR imaging of pineal cysts: Implications for natural history and follow-up. AJR Am J Roentgenol. 2001. 176: 737-43
5. Berhouma M, Ni H, Vallee B. The endoscopic intraventricular management of pineal cysts: A minimally invasive modus operandi. Acta Neurochir (Wien). 2013. 155: 1901-5
6. Bezuidenhout AF, Kasper EM, Baledent O, Rojas R, Bhadelia RA. Relationship between pineal cyst size and aqueductal CSF flow measured by phase contrast MRI. J Neurosurg Sci. 2021. 65: 63-8
7. Buysse DJ, Reynolds CF, Kupfer DJ, Thorpy MJ, Bixler E, Manfredi R. Clinical diagnoses in 216 insomnia patients using the International Classification of sleep disorders (ICSD), DSM-IV and ICD-10 categories: A report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994. 17: 630-7
8. D’Amico D, Usai S, Grazzi L, Rigamonti A, Solari A, Leone M. Quality of life and disability in primary chronic daily headaches. Neurol Sci. 2003. 24: S97-100
9. Davidson L. Endoscopic management of pineal cyst-associated aqueductal stenosis. Acta Neurochir (Wien). 2020. 162: 2975-82
10. Eide PK, Ringstad G. Results of surgery in symptomatic non-hydrocephalic pineal cysts: Role of magnetic resonance imaging biomarkers indicative of central venous hypertension. Acta Neurochir (Wien). 2017. 159: 349-61
11. El Damaty A, Fleck S, Matthes M, Baldauf J, Schroeder HWS. Pineal cyst without hydrocephalus: Clinical presentation and postoperative clinical course after infratentorial supracerebellar resection. World Neurosurg. 2019. 129: e530-7
12. Fain JS, Tomlinson FH, Scheithauer BW, Parisi JE, Fletcher GP, Kelly PJ. Symptomatic glial cysts of the pineal gland. J Neurosurg. 1994. 80: 454-60
13. Fathi AR, Eshtehardi P, Meier B. Patent foramen ovale and neurosurgery in sitting position: A systematic review. Br J Anaesth. 2009. 102: 588-96
14. Harrison EA, Mackersie A, McEwan A, Facer E. The sitting position for neurosurgery in children: A review of 16 years’ experience. Br J Anaesth. 2002. 88: 12-7
15. Jussila MP, Olsén P, Salokorpi N, Suo-Palosaari M. Follow-up of pineal cysts in children: Is it necessary?. Neuroradiology. 2017. 59: 1265-73
16. Kalani MY, Wilson DA, Koechlin NO, Abuhusain HJ, Dlouhy BJ, Gunawardena MP. Pineal cyst resection in the absence of ventriculomegaly or Parinaud’s syndrome: Clinical outcomes and implications for patient selection. J Neurosurg. 2015. 123: 352-6
17. Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Garber WH, Batenhorst A. A six-item short-form survey for measuring headache impact: The HIT-6. Qual Life Res. 2003. 12: 963-74
18. Krieg SM, Slawik H, Meyer B, Wiegand M, Stoffel M. Sleep disturbance after pinealectomy in patients with pineocytoma WHO I. Acta Neurochir (Wien). 2012. 154: 1399-405
19. Lipton RB, Liberman JN, Kolodner KB, Bigal ME, Dowson A, Stewart WF. Migraine headache disability and health-related quality-of-life: A population-based case-control study from England. Cephalalgia. 2003. 23: 441-50
20. Májovský M, Netuka D, Beneš V. Clinical management of pineal cysts: A worldwide online survey. Acta Neurochir (Wien). 2016. 158: 663-9
21. Májovský M, Netuka D, Beneš V. Conservative and Surgical treatment of patients with pineal cysts: Prospective case series of 110 patients. World Neurosurg. 2017. 105: 199-205
22. Májovský M, Netuka D, Beneš V. Is surgery for pineal cysts safe and effective? Short review. Neurosurg Rev. 2018. 41: 119-24
23. Májovský M, Řezáčová L, Sumová A, Pospíšilová L, Netuka D, Bradáč O. Melatonin and cortisol secretion profile in patients with pineal cyst before and after pineal cyst resection. J Clin Neurosci. 2017. 39: 155-63
24. Masina R, Ansaripour A, Beneš V, Berhouma M, Choque-Velasquez J, Eide PK. Surgical treatment of symptomatic pineal cysts without hydrocephalus-meta-analysis of the published literature. Acta Neurochir (Wien). 2022. 164: 61-77
25. Matjasko J, Petrozza P, Cohen M, Steinberg P. Anesthesia and surgery in the seated position: Analysis of 554 cases. Neurosurgery. 1985. 17: 695-702
26. Milton CK, Pelargos PE, Dunn IF. Headache outcomes after surgery for pineal cyst without hydrocephalus: A systematic review. Surg Neurol Int. 2020. 11: 384
27. Nevins EJ, Das K, Bhojak M, Pinto RS, Hoque MN, Jenkinson MD. Incidental pineal cysts: Is surveillance necessary?. World Neurosurg. 2016. 90: 96-102
28. Osborn AG, Preece MT. Intracranial cysts: Radiologicpathologic correlation and imaging approach. Radiology. 2006. 239: 650-64
29. Pastel DA, Mamourian AC, Duhaime AC. Internal structure in pineal cysts on high-resolution magnetic resonance imaging: Not a sign of malignancy. J Neurosurg Pediatr. 2009. 4: 81-4
30. Pitskhelauri DI, Konovalov AN, Abramov IT, Danilov GV, Pronin IN, Alexandrova EV. Pineal cyst-related aqueductal stenosis as cause of intractable headaches in nonhydrocephalic patients. World Neurosurg. 2019. 123: e147-55
31. Pitskhelauri DI, Konovalov AN, Kornienko VN, Serova NK, Arutiunov NV, Kopachev DN. Intraoperative direct third ventriculostomy and aqueductal stenting in deep-seated midline brain tumor surgery. Neurosurgery. 2009. 64: 256-66
32. Swinkin E, Bui E. Teaching neuroimages: Acute Parinaud syndrome. Neurology. 2017. 88: e164-5
33. Wisoff JH, Epstein F. Surgical management of symptomatic pineal cysts. J Neurosurg. 1992. 77: 896-900
34. Yeung JT, Young IM, Profyris C, Katsos K, Sughrue ME, Teo C. Resection of symptomatic pineal cysts provides durable clinical improvement: A breakdown of presenting symptoms and lessons learned. World Neurosurg. 2021. 150: e668-74
35. Yu M, Wang SM.editors. Neuroanatomy, Interstitial Nucleus of Cajal. Treasure Island, FL: StatPearls Publishing; 2022. p.