- Department of Neurological diseases and Neurosurgery, Peoples Friendship University of Russia (RUDN University), Moscow, Russia.
- Department of Pathological Anatomy, Morozov Children’s City Clinical Hospital, Moscow, Russia.
- Department of Neurosurgery, Morozov Children’s City Clinical Hospital, Moscow, Russia.
Correspondence Address:
Gerald Musa, Department of Neurological diseases and Neurosurgery, Peoples Friendship University of Russia (RUDN University), Moscow, Russia.
DOI:10.25259/SNI_312_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ilya O. Nosov1, Alexei N. Kislyakov2, Matvey I. Livshits3, Pavel V. Lobankin3, Gennady E. Chmutin1, Gerald Musa1. Pineal region neuroenteric cyst in a 13-year-old girl: A rare localization with postoperative recurrence and local dissemination. 01-Jul-2022;13:287
How to cite this URL: Ilya O. Nosov1, Alexei N. Kislyakov2, Matvey I. Livshits3, Pavel V. Lobankin3, Gennady E. Chmutin1, Gerald Musa1. Pineal region neuroenteric cyst in a 13-year-old girl: A rare localization with postoperative recurrence and local dissemination. 01-Jul-2022;13:287. Available from: https://surgicalneurologyint.com/surgicalint-articles/11684/
Abstract
Background: Neuroenteric cysts are rare cystic benign neoplasms of the central nervous system most often located in the spinal cord and rarely, intracranially. The nonspecific neuroimaging features make management planning potentially challenging. We present a case of a radiologically misdiagnosed neurenteric cyst with a complicated course.
Case Description: A 13-year-old girl presented with a 6-month history of headache, tinnitus, and dizziness. Initial magnetic resonance images (MRIs) were indistinguishable from a pineal arachnoid cyst with aqueductal stenosis and hydrocephalus. Cyst fenestration was performed through an infratentorial supracerebellar approach. Histology revealed a neurenteric cyst. On day 10 postoperatively, she deteriorated with acute hydrocephalus and cyst enlargement. An external ventricular drain was inserted. She remained asymptomatic thereafter. At 1-year postoperative, the patient remains asymptomatic despite the MRI showing cyst enlargement and local dissemination in the form of multiple cystic lesions in the cerebellum along the operative corridor. The patient was managed conservatively considering adhesion noted intraoperatively.
Conclusion: Neuroimaging features of brain cystic lesions may be nonspecific. Special attention should be awarded to posterior fossa and paramedian cystic lesions. Rarer lesions like neurenteric cysts should also be considered. When in doubt, we recommend using the following methods to prevent the free outflow of the cyst contents into the subarachnoid space: lining the cyst and operative corridor with cotton wool and puncture opening and suctioning of fluid. However, the “gold standard” remains surgical treatment with radical excision of the endodermal cyst capsule. It is necessary to preassess the possibility of total or subtotal resection.
Keywords: Arachnoid cyst, Brain embryology, Cyst recurrence, Endodermal cyst, Neurenteric cyst, Pineal region cyst
INTRODUCTION
Endodermal (neurenteric) cysts are rare benign neoplasms of the central nervous system, commonly associated with the spinal cord and considered rare when found intracranially. The approach to their treatment is ambiguous, which, together with the lack of convincing pathognomonic diagnostic features, makes attaining good neurosurgical outcomes a great challenge. A characteristic intracranial localization of neurenteric cysts is the posterior cranial fossa, that is, the ventral surface of the brain stem and cerebellopontine angle. Supratentorial location is less common. In other cases, they have been reported in the craniovertebral junction region and the cervicothoracic part of the spinal cord. The ratio of spinal and intracranial enterogenic cysts is 3:1.[
This paper presents an initially misdiagnosed and surgically treated intracranial endodermal cyst, leading to recurrence and local dissemination in a child.
CASE DESCRIPTION
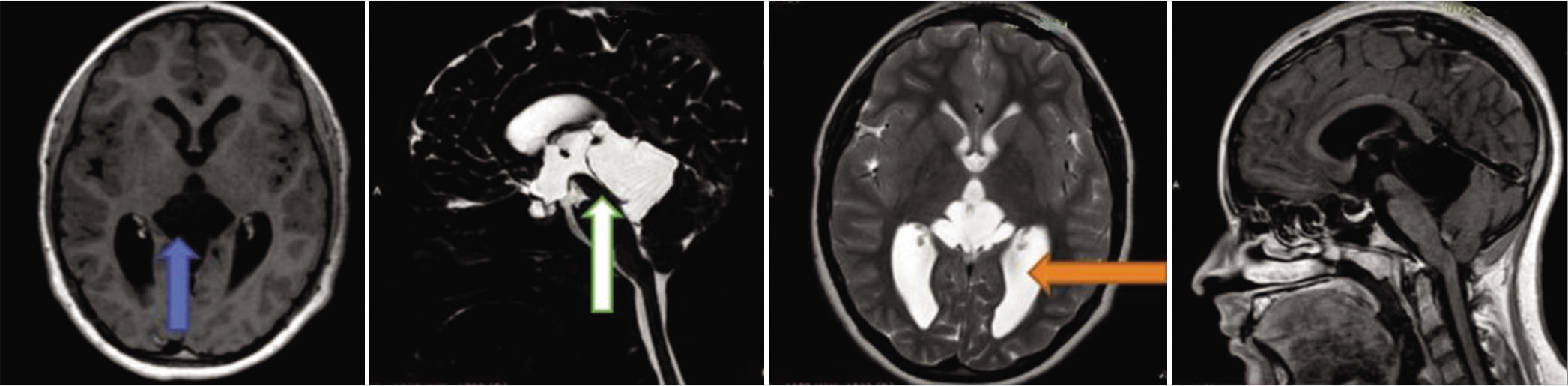
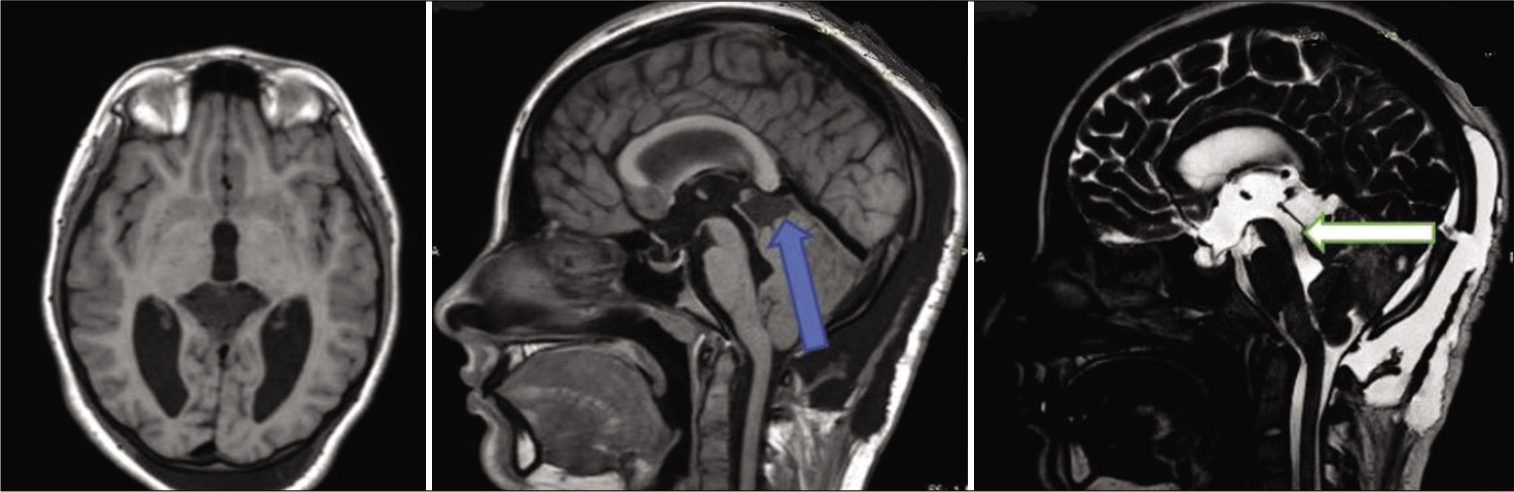
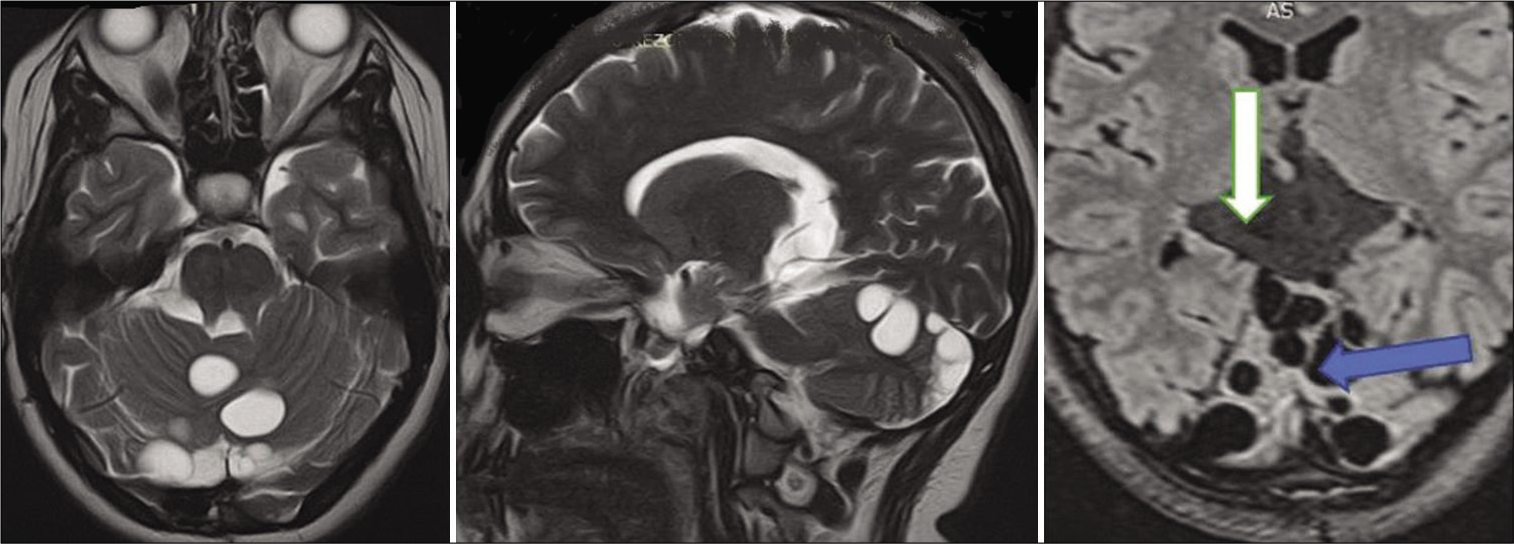
A 13-year-old girl presented with a 6-month history of headaches, tinnitus, and dizziness. The symptoms were partly relieved by nonsteroidal anti-inflammatory drugs. The attending neurologist requested a magnetic resonance imaging (MRI) of the brain which showed noncommunicating hydrocephalus secondary to a large pineal region cyst compressing the cerebellum and the cerebral aqueduct. Ophthalmology examination revealed features of papilledema. The child was fully conscious without focal or meningeal signs and symptoms. A brain MRI with contrast enhancement showed an irregular noncontract enhancing cystic lesion of the pineal region [
The cyst pushed the quadrigeminal plate and the aqueduct anteriorly and the cerebellum downward. Its lower-ventral surface adjoined the fourth ventricle, superiorly the walls of the cyst bordered on the bodies of moderately dilated lateral ventricles. Periventricular edema was noted. A diagnosis of arachnoid cyst of the pineal region with sub-compensated occlusive hydrocephalus was made. After multidisciplinary consultation, the patient was scheduled for microsurgical fenestration of the cyst walls and allows communication with the subarachnoid spaces of the posterior fossa to eliminate the mass effect and restore adequate CSF circulation.
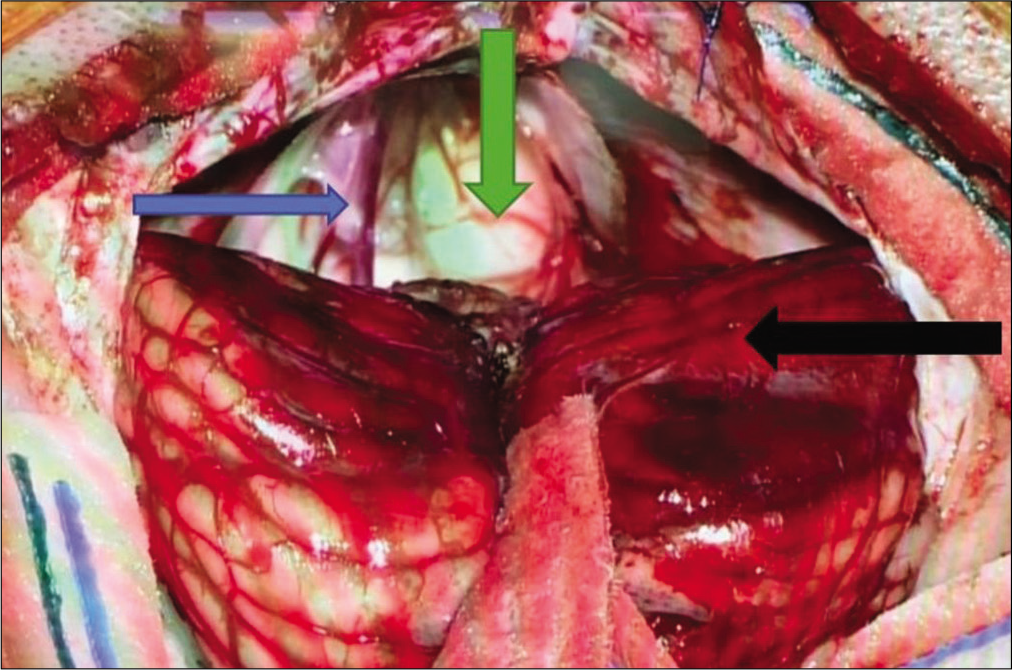
The cyst was approached through a midline infratentorialsupracerebellar corridor. On dural opening, a bulging cerebellum compressed by the cyst was noted with many arachnoid adhesions with the surrounding structures. These adhesions were carefully released and the whitish-yellow cyst was visualized [
The cystic fluid was partly cloudy and colorless. A biopsy of the capsule was taken. Postoperative computed tomography (CT) showed decreased cyst size with hyperdense content (interpreted as normal postoperative changes), mild pneumocephalus, and a patent cerebral aqueduct [
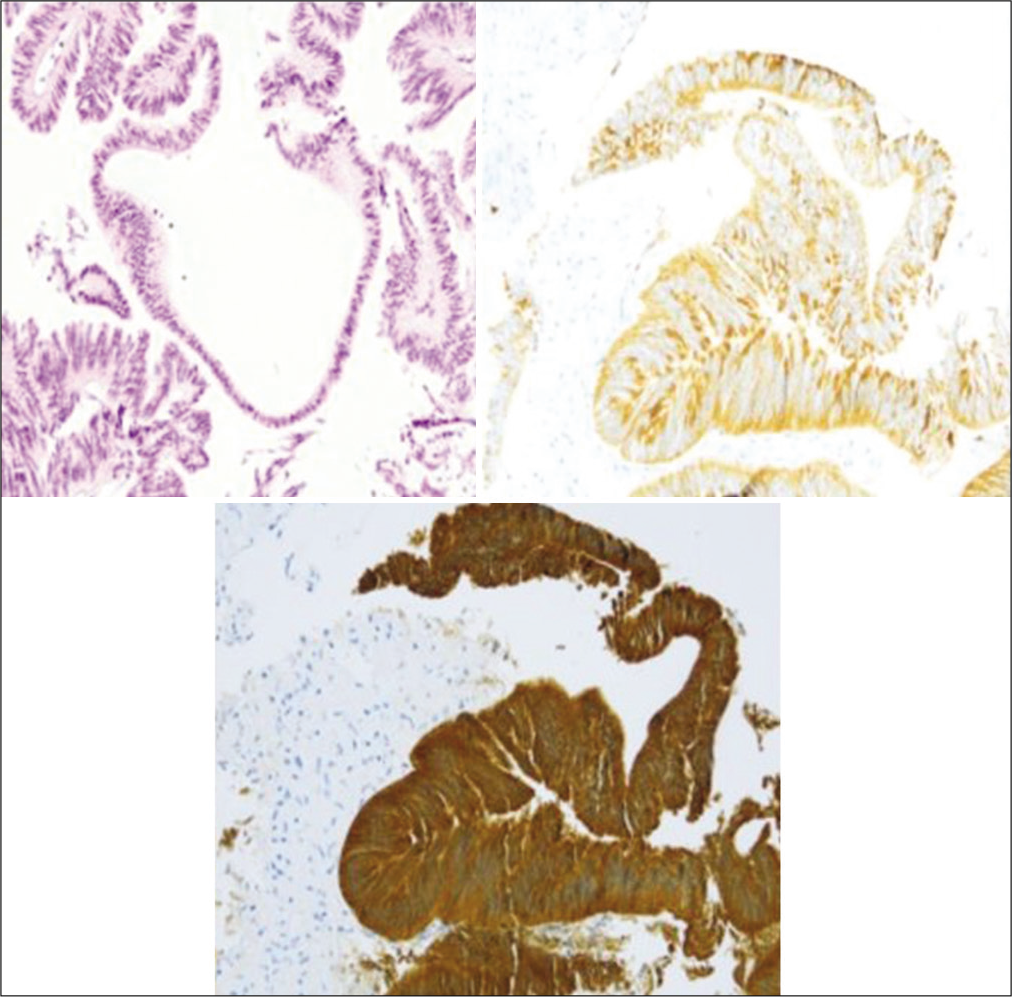
Pathohistological results indicated that the lesion was an endodermal cyst. The tissues showed dense and loose fibrous connective tissue lined by a single-layered and pseudostratified ciliated epithelium. Epithelial cells expressed cytokeratin (CK) 7, pan-keratin, and epithelial membrane antigen and were negative for glial fibrillary acidic protein, S100, and CK 20 [
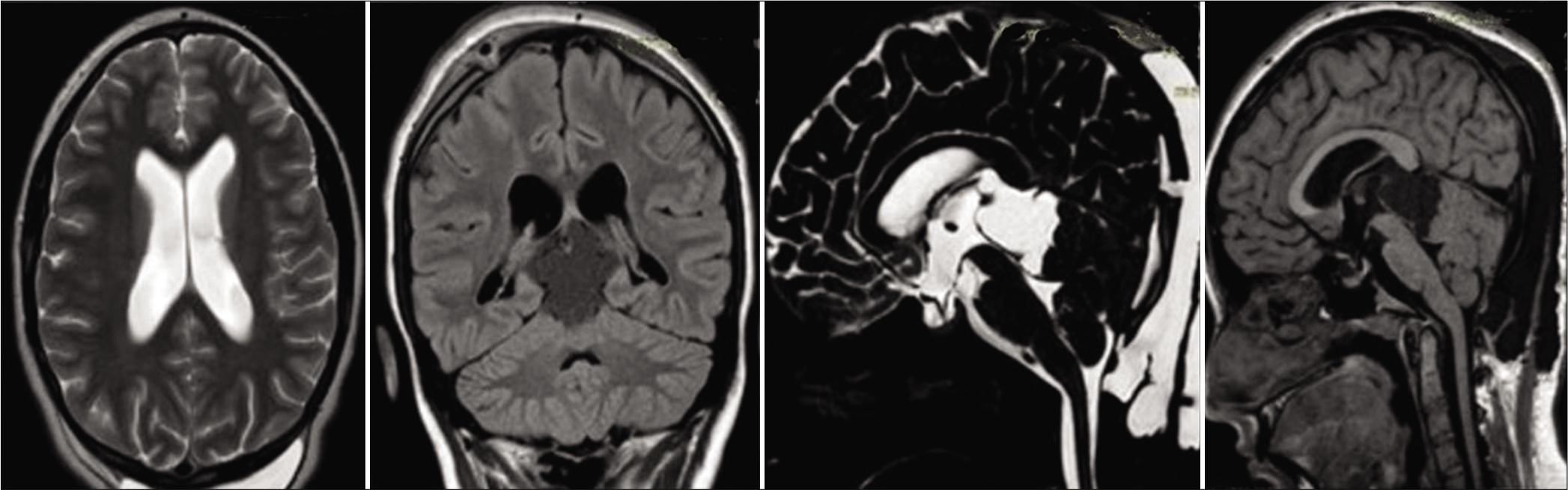
Day 6 postoperative MRI showed a decrease in the cyst size to 10–20% of the preoperative size with a symmetrical reduction in lateral ventricle size [
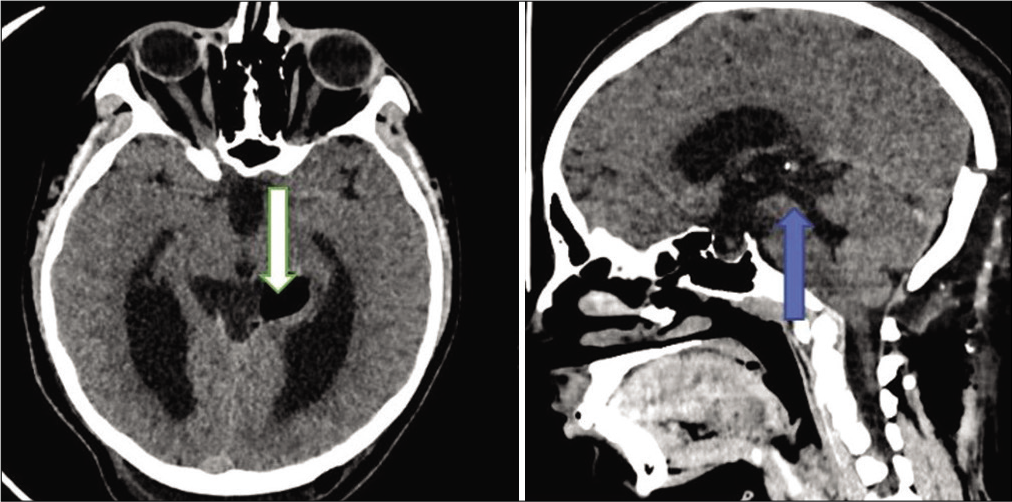
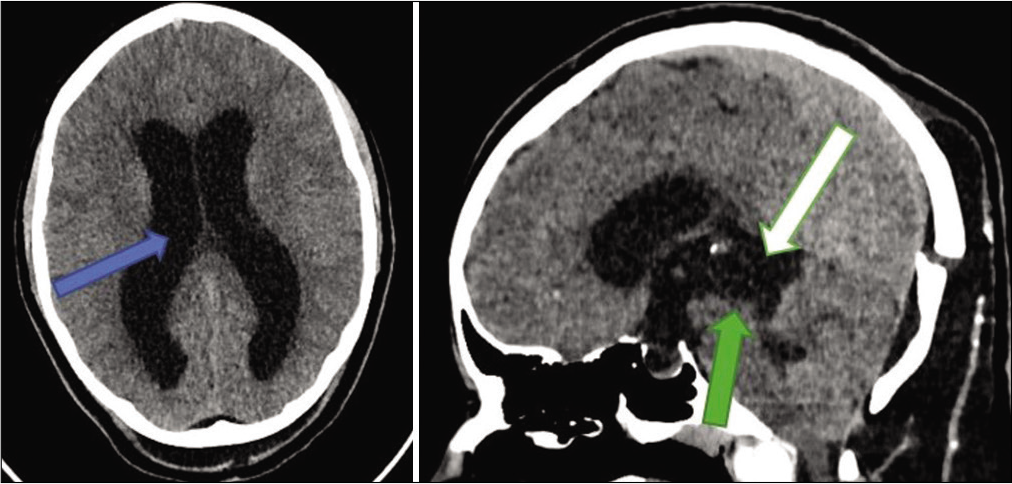
On day 10 postoperatively, the patient deteriorated with severe headache, vomiting, and developed a CSF leak. Control brain CT showed features of noncommunicating hydrocephalus and a × 2.5 increase in the cyst size compressing the cerebral aqueduct [
Figure 6:
Day 10 CT scan showing features of hydrocephalus, that is, ventricular enlargement, compression of the basal cisterns, and convexity subarachnoid spaces (blue arrow). Compared to previous images, the cyst is obviously enlarged (white arrow) with compression of the cerebral aqueduct (green arrow).
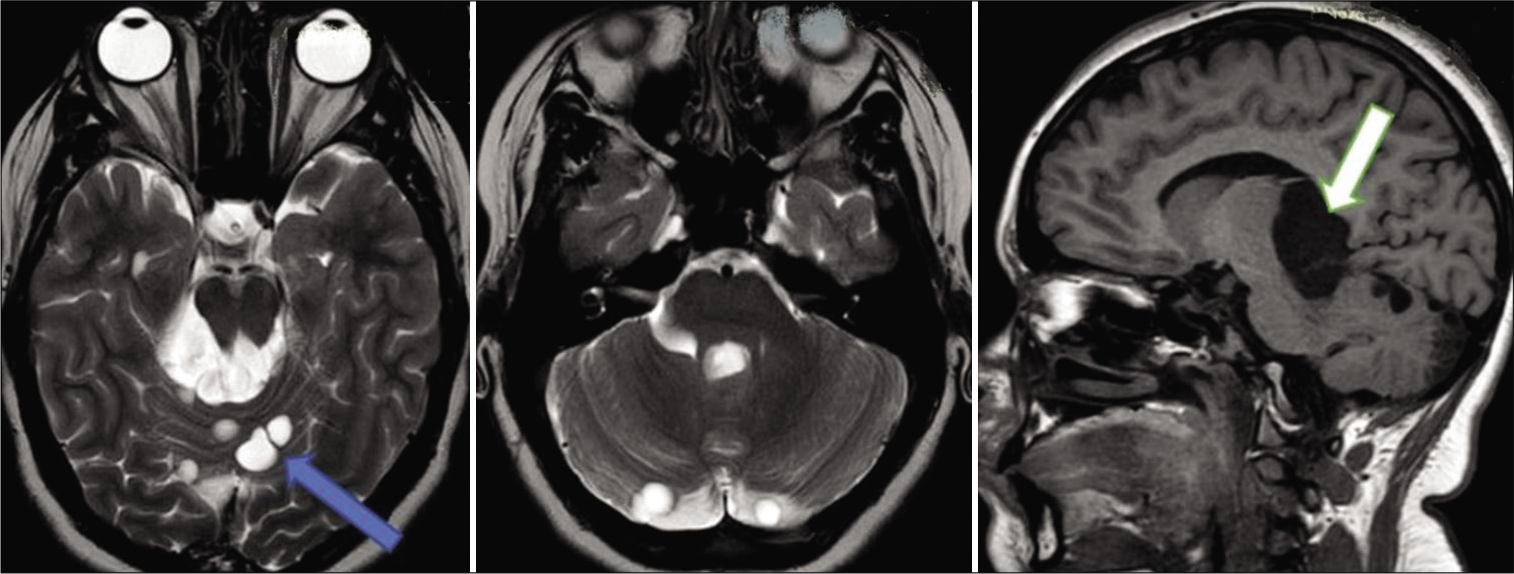
On day 4 after the second operation, she was discharged with no neurological deficit. At 1 and 3 months after discharge, the patient was still asymptomatic. Control brain MRIs were essential without change. However, 1-year routine control MRI showed significant changes compared to previous images [
On the superior surface of the cerebellum along the operative corridor of the supracerebellar approach, multiple noncommunicating cysts of various sizes were visualized. The size of the original pineal region cyst had slightly increased. Given the unusual progression of the disease, an enhanced MRI of the entire central nervous system and a brain positron emission tomography-CT with methionine was performed and normal. The patient was managed conservatively with a follow-up MRI done after 1 year and 20 months [
From the time of discharge, the patient has been asymptomatic and active with no focal neurological deficits. However, the authors are watchfully waiting and following up the patient clinically and radiologically. Should the cysts present with significant mass effect to cause clinical symptoms in the near future, surgical management with a goal of radical resection will be needed.
DISCUSSION
Endodermal cysts (neurenteric cysts and enterogenic cysts) are a rare malformation resulting from disorders of embryogenesis, leading to the formation of cystic cavities in the central nervous system filled with high-protein secretion.[
At the moment, there are no accurate data describing the time when cyst enlargement begins, the rate of growth, and when the patients present with clinical features. In the pediatric population, the average age of diagnosis is around 6.4 years.[
The treatment method of choice of neurenteric cysts is neurosurgical excision, which has shown favorable outcomes and prognosis. Total excision of the cyst and its capsule is the standard of surgical treatment and reduces the risk of recurrence of the enterogenic cyst.[
In some cases, malignant transformation occurs, and in all sources in the literature, the histological diagnosis was adenocarcinoma.[
Given the satisfactory condition of the child at the time of discharge, there was no clinical indication for additional surgical intervention. It is impossible to predict the future appearance of additional “daughter” cysts, including in the spinal subarachnoid space, despite the absence of convincing signs of communication between the endodermal cyst and the foramen Magna cistern in postoperative images. It seems quite logical to have possible dissemination of the enterogenic cysts in the subarachnoid space when their contents are poured out during surgery. In this regard, an individualized surgical approach to avoid opening the contents of the cysts into the subarachnoid may be ideal. It is worth assessing the possibility of radical cyst excision in open surgical intervention and considering other management options for symptomatic relief if radical excision is impossible. Nonsurgical methods seem appropriate, given reports of spontaneous regression of the neurenteric cyst and neurological symptoms without surgical intervention.[
The possibility of total cyst excision should be discussed during preoperative planning.
CONCLUSION
Neuroimaging features of brain cystic lesions may be nonspecific. Special attention should be awarded to posterior fossa and paramedian cystic lesions. Rarer lesions like neurenteric cysts should also be considered. When in doubt, we recommend using the following methods to prevent the free outflow of the cyst contents into the subarachnoid space: lining the cyst and operative corridor with cotton wool and puncture opening and suctioning of fluid. However, the “gold standard” remains surgical treatment with radical excision of the endodermal cyst capsule. It is necessary to preassess the possibility of total or subtotal resection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anderson T, Kaufman T, Murtagh R. Intracranial neurenteric cyst: A case report and differential diagnosis of intracranial cystic lesions. Radiol Case Rep. 2020. 15: 2649-54
2. Baek WK, Lachkar S, Iwanaga J, Oskouian RJ, Loukas M, Oakes WJ. Comprehensive review of spinal neurenteric cysts with a focus on histopathological findings. Cureus. 2018. 10: e3379
3. Chaynes P, Bousquet P, Sol JC, Delisle MB, Richaud J, Lagarrigue J. Recurrent intracranial neurenteric cysts. Acta Neurochir (Wien). 1998. 140: 905-11
4. de Oliveira RS, Cinalli G, Roujeau T, Sainte-Rose C, Pierre-Kahn A, Zerah M. Neurenteric cysts in children: 16 consecutive cases and review of the literature. J Neurosurg. 2005. 103: 512-23
5. Falsaperla R, Vitaliti G, Mauceri L, Romano C, Pavone P, Motamed-Gorji N. Levetiracetam in neonatal seizures as first-line treatment: A prospective study. J Pediatr Neurosci. 2017. 12: 24-8
6. Fujimoto T. Changes in cervical neurenteric cyst size. Case Rep Orthop Res. 2020. 3: 57-61
7. Fujisawa N, Oya S, Higashi M, Matsui T. Malignant transformation of a neurenteric cyst in the posterior fossa presenting with intracranial metastasis: A case report and literature review. NMC Case Rep J. 2015. 2: 123-7
8. Gauden AJ, Khurana VG, Tsui AE, Kaye AH. Intracranial neuroenteric cysts: A concise review including an illustrative patient. J Clin Neurosci. 2012. 19: 352-9
9. Kimura H, Nagatomi A, Ochi M, Kurisu K. Intracranial neurenteric cyst with recurrence and extensive craniospinal dissemination. Acta Neurochir (Wien). 2006. 148: 347-52
10. Krivoshapkin A, Sergeev G, Gaytan A, Salim N, Krotenkova I, Savitskiy I. A case report: Malignant transformation of a neurenteric cyst with intracranial metastases mimicking neurocysticercosis. Interdiscip Neurosur. 2021. 25: 101193
11. Lippman CR, Arginteanu M, Purohit D, Naidich TP, Camins MB. Intramedullary neurenteric cysts of the spine. Case report and review of the literature. J Neurosurg. 2001. 94: 305-9
12. Nicholas JE, Neil K, Francesco S, Gerald BB. Neurenteric cyst of the cerebellopontine angle: Case report. Neurosurgery. 1998. 42: 655-8
13. Okabe H, Katsura K, Yamano T, Tenjin H, Nakahara Y, Ishida M. Mucinous adenocarcinoma arising from supratentorial intramedullary neuroenteric cyst with bronchopulmonary differentiation. Neuropathology. 2014. 34: 420-4
14. Osborn A, Blaser S, Salzman K.editors. Neurenteric Cyst. Diagnostic Imaging: Brain. Salt Lake City, Utah: Amirsys; 2004. p. 40-1
15. Perrini P, Rutherford SA, King AT, du Plessis D, Di Lorenzo N. Enterogenous cysts of the cerebellopontine angle: Short review illustrated by two new patients. Acta Neurochir (Wien). 2008. 150: 177-84
16. Preece MT, Osborn AG, Chin SS, Smirniotopoulos JG. Intracranial neurenteric cysts: Imaging and pathology spectrum. AJNR Am J Neuroradiol. 2006. 27: 1211-6
17. Sahara Y, Nagasaka T, Takayasu M, Takagi T, Hata N, Yoshida J. Recurrence of a neurenteric cyst with malignant transformation in the foramen magnum after total resection. Case report. J Neurosurg. 2001. 95: 341-5
18. Schwartz ES, Barkovich AJ.editors. Congenital anomalies of the spine. Pediatric Neuroimaging. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. p. 863-922
19. Surash S, Ismail A, Loughrey C, van Hille P. Malignant transformation of a neurenteric cyst in the posterior fossa following complete excision. Br J Neurosurg. 2009. 23: 458-61
20. Takahashi S, Morikawa S, Saruhashi Y, Matsusue Y, Kawakami M. Percutaneous transthoracic fenestration of an intramedullary neurenteric cyst in the thoracic spine with intraoperative magnetic resonance image navigation and thoracoscopy. J Neurosurg Spine. 2008. 9: 488-92
21. Yang T, Wu L, Deng X, Yang C, Fang J, Zhao L. Clinical characteristics and surgical outcomes of spinal intramedullary ependymal cysts. Acta Neurochir (Wien). 2014. 156: 269-75
22. Yasuda M, Nakagawa H, Ozawa H, Inukai C, Watabe T, Mizuno J. Disseminated neurenteric cyst. J Neurosurg Spine. 2008. 9: 382-6