- Department of Neurosurgery, New York Medical College, Valhalla, New York, USA
- NeuroVascular Institute, Westchester Medical Center, Valhalla, New York, USA
- Capital Institute for Neurosciences, Stroke and Cerebrovascular Center, Capital Health System, Trenton, NJ, USA
Correspondence Address:
Arthur Wang
Capital Institute for Neurosciences, Stroke and Cerebrovascular Center, Capital Health System, Trenton, NJ, USA
DOI:10.4103/2152-7806.198730
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Arthur Wang, Justin Santarelli, Michael F. Stiefel. Pipeline embolization device as primary treatment for cervical internal carotid artery pseudoaneurysms. 19-Jan-2017;8:3
How to cite this URL: Arthur Wang, Justin Santarelli, Michael F. Stiefel. Pipeline embolization device as primary treatment for cervical internal carotid artery pseudoaneurysms. 19-Jan-2017;8:3. Available from: http://surgicalneurologyint.com/surgicalint_articles/pipeline-embolization-device-as-primary-treatment-for-cervical-internal-carotid-artery-pseudoaneurysms/
Abstract
Background:Limited data exists on the durability and occlusion rate of treating extracranial cervical internal carotid artery pseudoaneurysms using the pipeline embolization device (PED) flow-diverting stent.
Methods:Three patients presenting with dissecting cervical internal carotid artery pseudoaneurysms were treated with the PED as the sole treatment modality.
Results:In all three patients, successful aneurysmal occlusion and parent vessel reconstruction occurred on immediate angiography and continued on 6-month follow-up. No immediate or delayed complications were seen, and all patients remained neurologically intact.
Conclusion:Complete aneurysmal occlusion and long-term angiographic occlusion can occur after PED treatment of cervical carotid pseudoaneurysms. In select patients, the PED can be a suitable primary treatment modality with good neurological outcome for cervical carotid pseudoaneurysms.
Keywords: Cervical carotid, dissection, pipeline embolization device, pseudoaneursym
INTRODUCTION
Cervical internal carotid artery dissections and pseudoaneurysms are not uncommon. Recent studies have demonstrated a prevalence of blunt cervical vascular injury at 1.2–1.6% of trauma patients and 2.7% of multisystem trauma patients.[
The majority of dissections, Denver Grades 1 and 2, heal with medical therapy and less than 10% progress to a pseudoaneurysm.[
The recent advances in endovascular technology have shifted treatment paradigms away from microsurgical techniques and towards endovascular treatment of dissecting carotid pseudoaneurysms.[
The application of the PED on cervical carotid pseudoaneurysms is limited. To date, there has only been one published study applying the PED to treat high cervical carotid pseudoaneurysms.[
CASE REPORTS
Three patients with Denver Grade 3, dissecting cervical carotid artery pseudoaneurysms were treated at our institution using the PED [
All patients were loaded on aspirin and clopidogrel 24 hours prior to PED placement with platelet reactivity testing performed to confirm adequate platelet inhibition. At the beginning of each case, a baseline activated clotting time (ACT) was obtained, and a weight based intraoperative bolus of intravenous heparin was given after femoral artery access to maintain an ACT of >200 seconds throughout the procedure. All interventions were performed in a biplanar fluoroscopic angiography suite under general anesthesia using intraoperative monitoring (somatosensory evoked potentials, motor evoked potentials, and electroencephalography).
The PED (ev3-Covidien) was used in all cases as the only device. Conventional and three-dimensional (3D) angiographic studies were performed and working angle projections for PED placement were acquired at high magnification of the cervical carotid artery. In all patients, consecutive PED stents were placed in a telescoping fashion, distal to proximal, to cover the pseudoaneurysm.
Immediately after the treatment, all patients were extubated and observed in the intensive care unit for 24 hours with hourly neurological examinations. All patients were verified to be aspirin and clopidogrel responders and were continued on daily 325 mg aspirin and 75 mg clopidogrel.
The angiographic features of the pseudoaneurysms and outcomes with respect to clinical and radiographic images of the patients are reviewed. With radiographic outcome, immediate and 6-month follow up angiograms of the patients were reviewed to examine contrast stagnation, occlusion of the pseudoaneurysm, and in-stent patency following PED deployment.
Case 1
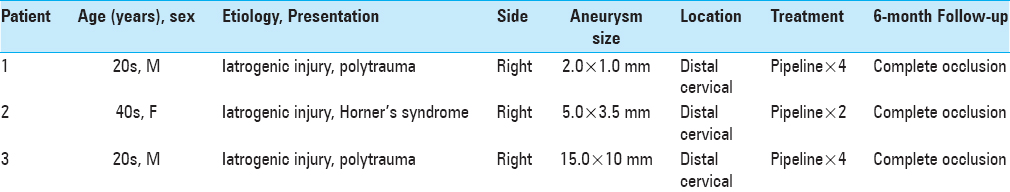
Patient 1, in the second decade of life, presented intubated with multisystem trauma from a motor vehicle accident; GCS 5T and an injury severity score (ISS) of 41/75. Initial CT angiogram of the head and neck demonstrated grade I mild focal narrowing of the right cervical carotid artery at the level of C1-2. A repeat CT angiogram 1 week later showed stability of the diseased carotid segment whereas a CT angiogram at 3 weeks demonstrated interval development of a 6.5 mm × 14 mm dissecting pseudoaneurysm of the right cervical internal carotid artery at the C1-2 level [
Figure 1
(a) CT angiogram (CTA) of the head and neck showing the diseased cervical carotid artery segment with pseudoaneurysm formation, dissection flap, and intervening arterial stenosis. (b) Lateral and (c) AP view digital subtraction angiograms (DSA) before deployment of the pipeline embolization device (PED) show a high cervical carotid pseudoaneurysm and vessel stenosis. (d) Immediate DSA following PED placement shows improved vessel caliber. (e) CTA post-treatment demonstrating the span of the telescoping PED stents and immediate improvement in vessel caliber and aneurysm occlusion. (f) DSA at 6-month follow-up showing the pseudoaneurysm remaining occluded and normal contour of the cervical carotid artery
Case 2
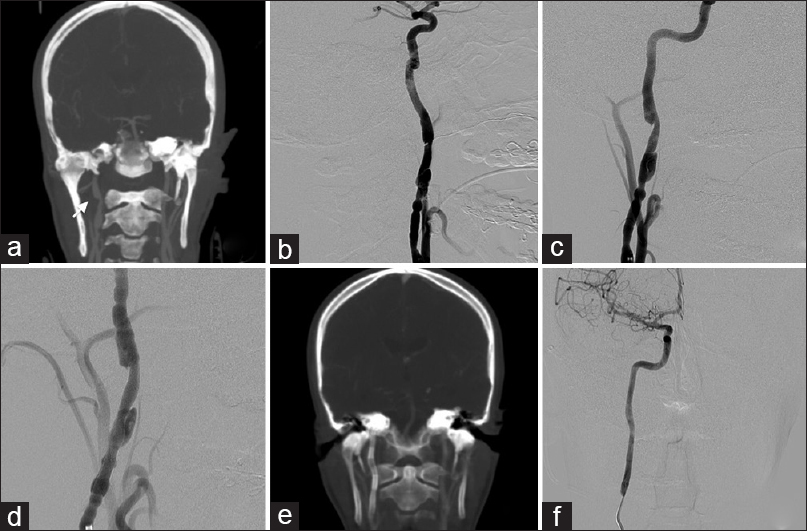
Patient 2, in the fourth decade of life, presented with clinical symptoms consistent with recurrent Horner's syndrome after recovering from an upper respiratory infection. CT angiogram of the neck demonstrated an enlarging medially projecting 3.3 mm × 6.6 mm pseudoaneurysm of the right cervical internal carotid artery compared to the CT angiogram 3 years ago [
Figure 2
(a) CTA of the head and neck demonstrates a pseudoaneurysm of the high cervical carotid artery. (b) Oblique view DSA before deployment of the PED shows a cervical carotid pseudoaneurysm. (c) Immediate and (d) 6-month follow-up DSA show decreased aneurysm filling, intra-aneurysmal stasis, and complete occlusion of the pseudoaneurysm
Case 3
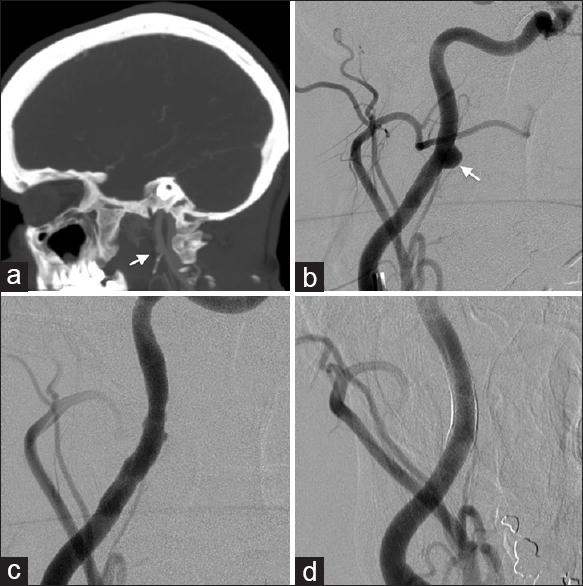
Patient 3, in the second decade of life, presented intubated with multisystem trauma from a motor vehicle accident; GCS 6T with extensive extremity fractures and lung injuries with an ISS of 48/75. Initial CT angiogram of the head and neck demonstrated a dissection flap with associated pseudoaneurysm of the right distal cervical internal carotid artery. Follow up CTA showed that the pseudoaneurysm enlarged [
Figure 3
(a) CTA of the head and neck demonstrates a pseudoaneurysm of the high cervical carotid artery. (b) Lateral and (c) AP view DSA before deployment of the PED shows a cervical carotid pseudoaneurysm. (d) Immediate DSA shows decreased aneurysm filling. (e) CTA post-treatment demonstrating the PED stents. (f) DSA at 6-month follow-up showing the pseudoaneurysm remaining occluded and normal contour of the cervical carotid artery
PEDs were successfully placed in all three patients. In patients 1 and 3, overlapping PEDs were deployed. In patient 2, a second PED was needed after foreshortening of the first PED was observed. At the completion of the cases, increased intra-aneurysmal contrast stagnation, improved vessel caliber, and normal intracranial vascular flow were seen on the final angiographic runs.
No neurological deficits or access site complications were observed. Patients 1 and 3 underwent multiple surgeries to repair severe facial and lower extremity fractures and remained neurologically intact at the time of discharge. Patient 2 continued to exhibit Horner's syndrome at the time of discharge. All patients were discharged on daily aspirin and clopidogrel therapy.
Patient 1 returned within 1 week of discharge with transient left-sided weakness. A CT head confirmed no delayed intracerebral hemorrhage, and a CT angiogram demonstrated patent intracranial vessels without in-stent thrombosis. Magnetic resonance imaging of the brain showed no restricted diffusion changes. The patient was observed and discharged after complete resolution of his symptoms.
All patients remained neurologically intact (modified rankin scale = 0) at 6-month follow up visit. Complete aneurysm occlusion and normal vessel caliber were seen on repeat angiography at the time [Figures
DISCUSSION
The majority of extracranial dissections, Denver grade 1 and 2, heal with medical therapy alone, however, medical therapy is not as effective for pseudoaneurysms, Denver Grade 3.[
Treatment of extracranial carotid artery pseudoaneurysms
Stent-assisted coil embolization and endovascular stent reconstruction are effective means of treating internal carotid pseudoaneurysms. Bush et al. reported their success with stent-assisted coiling of five pseudoaneurysms with four of five stent constructs remaining patent on follow-up.[
Stenting alone is an alternative treatment. A study by Yi et al. demonstrated a 100% occlusion rate of ten carotid pseudoaneurysms treated with self-expanding or balloon-expandable covered stents without recanalization at 6 months.[
The ideal device for the craniocervical junction would be self-expanding and flexible enough to adapt to arterial walls, atraumatic to the vessel wall upon deployment, and have enough radial force to treat the diseased segment. Pham et al. conducted a systematic review and found that 15 of 28 studies reported the successful use of the self-expandable Wall stent (Stryker Neurovascular, Fremont, CA, USA) and another four studies with the self-expandable SMART stent (Cordis, Miami, Fl, USA) in treating high cervical carotid pseudoaneurysms.[
Current applications of the pipeline embolization device for cervical and intracranial dissections
The PED is a microcatheter delivered self-expanding stent-like construct that is very effective for treating intracranial fusiform, large, or wide-necked aneurysms. The PED offers the advantage of being used as a stand-alone device with immediate flow diversion and intra-aneurysmal thrombosis.
The application of the PED on treating dissecting pseudoaneurysms has mainly focused on the intracranial circulation.[
The PED has several properties that make it suitable for treating extracranial cervical dissections. Its flexible self-expanding design combined with the advantage of using a smaller guide and microcatheter platform allows for greater maneuverability through tortuous and narrowed vessel segments at the skull base. In their series of 11 patients with 13 extracranial carotid dissections, Brzezicki et al. demonstrated immediate complete revascularization in 91% of the vessels and complete occlusion of 50% of the associated pseudoaneurysms.[
Technical considerations in choosing a stand-alone construct
Stent reconstruction in the distal cervical carotid artery may be challenging because of the tortuosity of this segment and the changing diameter and mobility of the cervical carotid artery with head movements. There has been limited use of the PED on extracranial cervical carotid pseudoaneurysms. In our three cases, conventional self-expanding carotid or smaller biliary stents would have necessitated larger constructs being navigated through the dissected segment and into the petrous carotid segment. The PED's flexible and self-expanding design combined with the advantage of using a smaller guide and microcatheter platform allows for greater maneuverability through tortuous and narrowed vessel segments.[
The length and diameter of the diseased segment as well as the adjacent normal segments are carefully evaluated when choosing the appropriate stent or flow diverter construct. Stents such as the Neuroform and Enterprise (Codman Neurovascular, Raynham, MA, USA) have a maximum diameter of 4.5 mm, making them less suitable in the larger mid-cervical and proximal-cervical internal carotid artery. The PED is available from 2.5 to 5.0 mm with 0.25 mm increments and lengths of 10 to 35 mm. The 5.0 mm PED expands to 5.25 mm.[
It is important to consider that oversizing the PED can lead to elongation of the device with poor coverage of the lesion and a decrease in flow diversion. Undersizing the device may result in poor wall apposition, an endoleak, as well as a potential risk of stent migration. Selection of a suboptimal PED and potential stent foreshortening and delayed migration of the device in both intracranial and extracranial disease has been reported.[
The effectiveness of flow diversion may vary with the amount of metal coverage. The high porosity of currently available stents limits the ability to change inflow within an aneurysm. The PED was specifically designed to address this issue with the goal of promoting more effective flow diversion. The PED achieves this because of its low porosity and 30–35% surface metal area coverage, which is more than other stents such as the Xpert Biliary Stent (Abbott Vascular, Santa Clara, CA, USA) (20%) and Neuroform (9%) and Enterprise Stents (7%) [
Lastly, while the PED offers certain advantages over other stents available in the market, it is associated with adverse cost considerations ($12500 per PED) and may not be ideal for each patient and each institution, especially if multiple PEDs will be placed.
CONCLUSIONS
The application of flow diverters in treating cervical carotid pseudoaneurysms is limited. Our study highlights the use of the PED as the sole modality for the treatment of cervical carotid artery dissecting pseudoaneurysms. Although small, our series demonstrates the successful use of the PED as a primary endovascular modality with excellent 6-month radiographic and clinical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amenta PS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH. Successful treatment of a traumatic carotid pseudoaneurysm with the Pipeline stent: Case report and review of the literature. Surg Neurol Int. 2012. 3: 160-
2. Ansari SA, Thompson BG, Gemmete JJ, Gandhi D. Endovascular treatment of distal cervical and intracranial dissections with the Neuroform stent. Neurosurgery. 2008. 62: 636-46
3. Benndorf G, Campi A, Schneider GH, Wellnhofer E, Unterberg A. Overlapping stents for treatment of a dissecting carotid artery aneurysm. J Endovasc Ther. 2001. 8: 566-70
4. Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: Implications of a new grading scale. J Trauma. 1999. 47: 845-53
5. Biffl WL, Ray CE, Moore EE, Mestek M, Johnson JL, Burch JM. Noninvasive diagnosis of blunt cerebrovascular injuries: A preliminary report. J Trauma. 2002. 53: 850-6
6. Biffl WL, Ray CE, Moore EE, Franciose RJ, Aly S, Heyrosa MG. Treatment-related outcomes from blunt cerebrovascular injuries: Importance of routine follow-up arteriography. Ann Surg. 2002. 235: 699-706
7. Brzezicki G, Rivet DJ, Reavey-Cantwell J. Pipeline Embolization Device for treatment of high cervical and skull base carotid artery dissections: Clinical case series. J Neurointer Surg. 2016. 8: 722-8
8. Bush RL, Lin PH, Dodson TF, Dion JE, Lumsden AB. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther. 2001. 8: 53-61
9. Chalouhi N, Satti SR, Tjoumakaris S, Dumont AS, Gonzalez LF, Rosenwasser R. Delayed migration of a pipeline embolization device. Neurosurgery. 2013. 72: Ons 229-34
10. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G. Spontaneous Delayed Migration/Shortening of the Pipeline Embolization Device: Report of 5 Cases. AJNR Am J Neuroradiol. 2013. 34: 2326-30
11. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Gonzalez LF, Randazzo C, Starke RM. Treatment of posterior circulation aneurysms with the Pipeline Embolization Device. Neurosurgery. 2013. 72: 883-9
12. Cohen JE, Gomori JM, Moscovici S, Bala M, Itshayek E. The use of flow diverter stents in the management of traumatic vertebral artery dissections. J Clin Neurosci. 2013. 20: 731-4
13. Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ. Treatment of posttraumtic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000. 48: 470-2
14. De Barros Faria M, Castro RN, Lundquist J, Scrivano E, Ceratto R, Ferrario A. The role of the pipeline embolization device for the treatment of dissecting intracranial aneurysms. AJNR Am J Neuroradiol. 2011. 32: 2192-5
15. Ducruet AF, Crowley RW, Albuquerque FC, McDougall CG. Reconstructive endovascular treatment of a ruptured vertebral artery dissecting aneurysm using the pipeline embolization device. J Neurointerv Surg. 2013. 5: e20-
16. Edwards NM, Fabian TC, Claridge JA, Timmons SD, Fischer PE, Croce MA. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: Results from longterm followup. J Am Coll Surg. 2007. 204: 1007-13
17. Fiorella D, Albuquerqu FC, Deshmukh VR, Woo HH, Rasmussen PA, Masaryk TJ. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006. 59: 291-300
18. Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct: Case report. Neurosurgery. 2010. 67: 313-4
19. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012. 54: 369-82
20. Fischer S, Perez MA, Kurre W, Albes G, Bäzner H, Henkes H. Pipeline embolization device for the treatment of intra- and extracranial fusiform and dissecting aneurysms: Initial experience and long-term follow-up. Neurosurgery. 2014. 75: 364-74
21. Hu YC, Chugh C, Mehta H, Stiefel MF. Early angiographic occlusion of ruptured blister aneurysms of the internal carotid artery using the Pipeline Embolization Device as a primary treatment option. J Neurointervent Surg. 2014. 6: 740-3
22. Jeon P, Kim BM, Kim DI, Shin YS, Kim KH, Park SI. Emergent self-expanding stent placement for acute intracranial or extracranial internal carotid artery dissection with significant hemodynamic insufficiency. AJNR. 2010. 31: 1529-32
23. Kadkhodayan Y, Jeck DT, Moran CJ, Derdeyn CP, Cross DT. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005. 26: 2328-35
24. Klisch J, Turk A, Turner R, Woo HH, Fiorella D. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. AJNR Am J Neuroradiol. 2011. 32: 627-32
25. Levitt MR, Ghodke BV, Hallam DK, Sekhar LN, Kim LJ. Incidence of microemboli and correlation with platelet inhibition in aneurysmal flow diversion. AJNR Am J Neuroradiol. 2013. 34: 2321-5
26. Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000. 21: 1280-92
27. Martin AR, Cruz JP, Matouk CC, Spears J, Marotta TR. The Pipeline Flow-Diverting Stent for Exclusion of ruptured Intracranial Aneurysms with Difficult Morphologies. Neurosurgery. 2012. 70: ons1-28
28. Narata AP, Yilmaz H, Schaller K, Lovblad KO, Pereira VM. Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery. 2012. 70: 982-8
29. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The Pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011. 32: 34-40
30. Nerva JD, Morton RP, Levitt MR, Osbun JW, Ferreira MJ, Ghodke BV. Pipeline Embolization Device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg. 2015. 7: 210-6
31. Park MS, Albuquerque FC, Nanaszko M, Sanborn MR, Moon K, Abla AA. Critical assessment of complications associated with use of the Pipeline Embolization Device. J Neurointerv Surg. 2015. 7: 652-9
32. Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, Batjer HH. Endovascular stenting of extracranial carotid and vertebral artery dissections: A systematic review of the literature. Neurosurgery. 2011. 68: 856-66
33. Prasad V, Gandhi D, Jindal G. Pipeline endovascular reconstruction of traumatic dissecting aneurysms of the intracranial internal carotid artery. J Neurointerv Surg. 2014. 6: e48-
34. Rahal JP, Dandamudi VS, Heller RS, Safain MG, Malek AM. Use of concentric Solitaire stent to anchor Pipeline flowdiverter constructs in treatment of shallow cervical carotid dissecting pseudoaneurysms. J Clin Neurosci. 2014. 21: 1024-8
35. Rosset E, Albertini JN, Magnan PE, Ede B, Thomassin JM, Branchereau A. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg. 2000. 31: 713-23
36. Seth R, Obuchowski AM, Zoarski GH. Endovascular Repair of Traumatic Cervical Internal Carotid Artery Injuries: A Safe and Effective Treatment Option. AJNR Am J Neuroradiol. 2013. 34: 1219-26
37. Yeung TW, Lai V, Lau HY, Poon WL, Tan CB, Wong YC. Long-term outcome of endovascular reconstruction with the Pipeline embolization device in the management of unruptured dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 2012. 116: 882-7
38. Yi AC, Palmer E, Luh GY, Jacobson JP, Smith DC. Endovascular treatment of Carotid and Vertebral Pseudoaneurysms with Covered Stents. AJNR Am J Neuroradiol. 2008. 29: 983-87
39. Zhou W, Lin PH, Bush RL, Peden E, Guerrero MA, Terramani T. Carotid artery aneurysm: Evolution of management over two decades. J Vasc Surg. 2006. 43: 493-6