- Department of Neurosurgery, Tokai University Hachioji Hospital, Ishikawamachi, Hachioji, Tokyo, Japan.
Correspondence Address:
Masaaki Imai, Department of Neurosurgery, Tokai University Hachioji Hospital, Ishikawamachi, Hachioji, Tokyo, Japan.
DOI:10.25259/SNI_969_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masaaki Imai, Azusa Sunaga, Rie Aoki, Takahiro Osada, Kaori Hoshikawa, Shinri Oda, Masami Shimoda. Possibility of arterial spin labeling perfusion magnetic resonance imaging sequences with steroid therapy for Tolosa-Hunt syndrome: A case report and review of literature. 20-Jan-2022;13:27
How to cite this URL: Masaaki Imai, Azusa Sunaga, Rie Aoki, Takahiro Osada, Kaori Hoshikawa, Shinri Oda, Masami Shimoda. Possibility of arterial spin labeling perfusion magnetic resonance imaging sequences with steroid therapy for Tolosa-Hunt syndrome: A case report and review of literature. 20-Jan-2022;13:27. Available from: https://surgicalneurologyint.com/surgicalint-articles/11349/
Abstract
Background: The diagnostic criteria for Tolosa-Hunt syndrome (THS) were updated in 2013 in the 3rd Edition of the International Classification of Headache Disorders. It is now possible to diagnose THS based on the presence of granulomatous inflammation demonstrated on magnetic resonance imaging (MRI) without confirmation by biopsy. No previous study has reported the use of arterial spin labeling (ASL) perfusion MRI for diagnosing THS. Here, we report a case of THS in which ASL was used in the initial identification and to monitor therapeutic response following steroid therapy.
Case Description: An 86-year-old man was complaining chiefly of the left orbital pain, as well as occipital pain, nausea, epiphora, and diplopia. Neurologically, his eye movements showed left adduction disorder and palsy of the right cranial nerve III. Magnetic resonance angiography revealed no abnormality in the left internal carotid artery. Contrast-enhanced MRI showed a region of slightly high signal in the left cavernous sinus. ASL was obtained using pCASL (TR/TE, 9000/98. 48 ms; postlabeling delay: 1525 ms; axial plane) revealed hyperperfusion from the intercavernous sinus to the vicinity of the left cavernous sinus due to a local increase in cerebral blood flow. The symptoms disappeared on day 62 of the treatment and he was in complete remission. Follow-up ASL was performed every other month showed reduced perfusion as the symptoms improved and confirmed the absence of a tumor over the follow-up period.
Conclusion: This simple technique will play an important role in confirming no recurrence after steroid therapy treatment.
Keywords: Arterial spin labeling, Headache, Magnetic Resonance Imaging, Steroid therapy, Tolosa-Hunt syndrome
INTRODUCTION
Tolosa-Hunt syndrome (THS) was first reported in 1954 by Tolosa and a similar case was reported by Hunt et al. in 1961. Smith and Taxdal first proposed the term THS in 1966.[
The diagnostic criteria for THS are unilateral orbital or periorbital pain; paralysis of one or more of the unilateral cranial nerves III, IV, or VI; and inflammation of granulomatous lesions within the cavernous sinus, superior orbital fissure, or orbit. “Demonstration of granulomas by biopsy” in the International Classification of Headache Disorders (ICHD)-2 criteria was revised to “Demonstration of granulomas by magnetic resonance imaging (MRI) or biopsy” in the ICHD-3 criteria.[
CASE PRESENTATION
Two months before presentation at our department, an 86-year-old man was complaining chiefly of the left orbital pain, as well as occipital pain, nausea, epiphora, and diplopia. He had a previous history of diabetes, diabetic nephropathy, diabetic retinopathy, hypertension, and dyslipidemia but no history of smoking or drinking. At the time of admission, no general physical abnormalities, conjunctival congestion, or exophthalmos were detected. Neurologically, his left eye was in frontal view with abduction, and the left pupillary light reflex was slow and nonmydriatic. His eye movements showed left adduction disorder and palsy of the left cranial nerve III.
The laboratory findings were as follows: blood count: WBC 10,400/μL ↑ ; biochemistry: CRP 0.010 mg/dL, Cre 1.16 mg/dL ↑, BS 104 mg/dL, HbA1c 7.4% ↑ ; and blood coagulation: D-dimer 2.1 μg/mL. Antinuclear antibodies were 40 times higher than normal; and anti-DNA antibodies were 2.0 IU/mL. There was no increase in CEA, AFP, CA19-9, SCC, PSA, or IL-2R tumor markers.
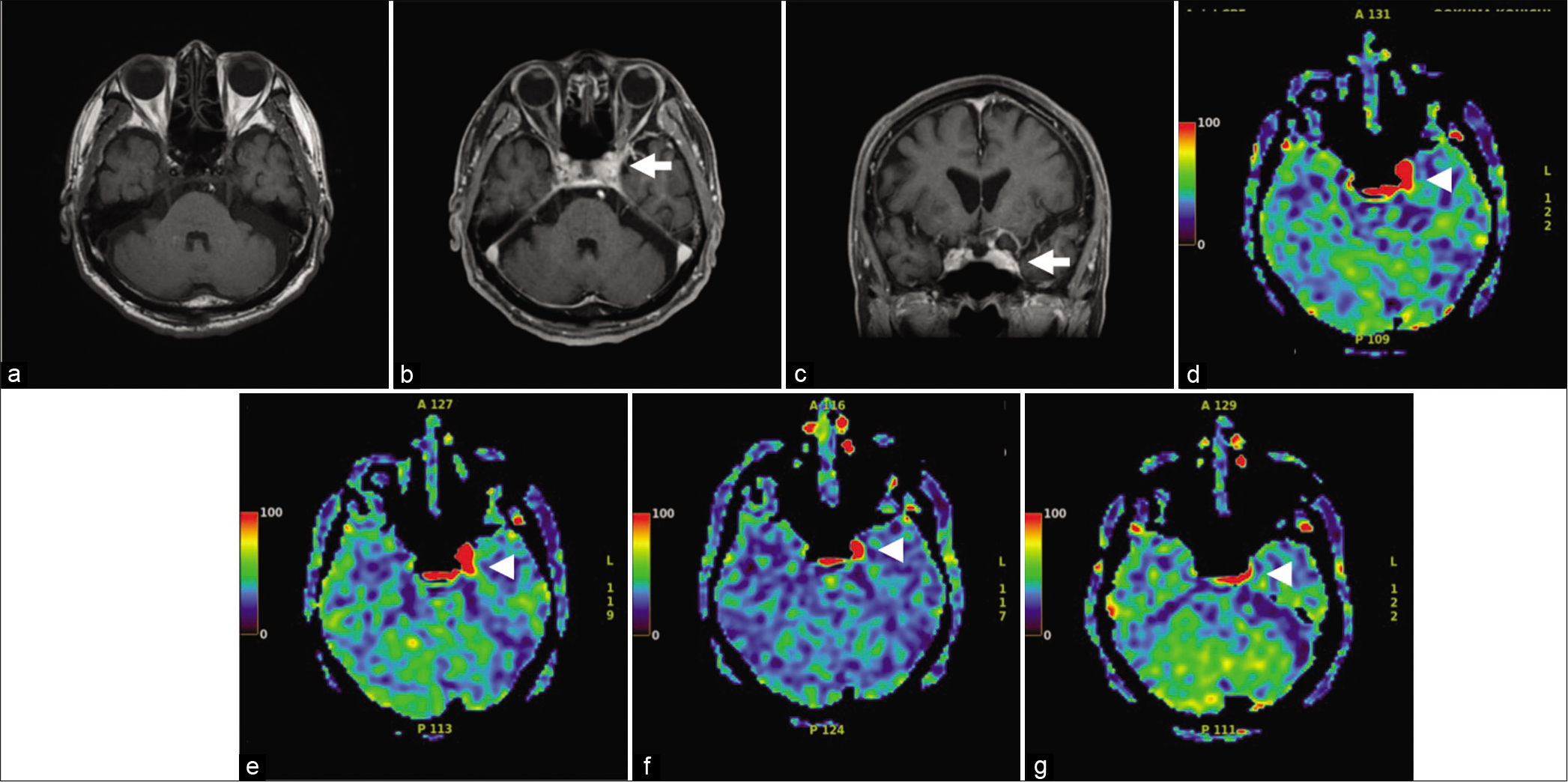
Uncontrasted T1WI obtained using a 1.5 T superconducting magnet (GE Healthcare) of the brain showed no abnormalities of the left cavernous sinus. Magnetic resonance angiography (MRA) revealed no abnormality in the left internal carotid artery. Contrast-enhanced MRI showed a region of slightly high signal in the left cavernous sinus. ASL was obtained using pCASL (TR/TE, 9000/98. 48 ms; postlabeling delay (PLD): 1525 ms, 2525 ms; axial plane) revealed hyperperfusion from the intercavernous sinus to the vicinity of the left cavernous sinus due to a local increase in CBF [
Figure 1:
(a) T1-weighted images (T1WI) magnetic resonance imaging (MRI) in the axial planes shows no abnormal findings. (b and c) Contrast-enhanced magnetic resonance imaging shows a region of slightly high signal in the left cavernous sinus (arrow). (d-g) Axial ASL demonstrates a focal increase in cerebral blood flow (arrowhead) from the intercavernous sinus to the vicinity of the left cavernous sinus due to a local increase in cerebral blood flow. (At onset (e), day 49 (f), day 62 (g), and 3 months (h)) after initiation of steroid therapy. Axial ASL demonstrates focal decrease of cerebral blood flow.
Clinical course
Ophthalmoplegic migraine and diabetic neuropathy were discounted because of the mass found in the cavernous sinus on MRI of the brain, and vascular lesions were ruled out because of the MRA findings. The possibility of sarcoidosis, Wegener’s granulomatosis, or metastatic brain tumor was considered low as tumor markers and chest CT showed no lung lesions. SLE was discounted as the anti-DNA antibody was not elevated. Accordingly, we made a diagnosis of THS.
As his diabetes was well controlled, treatment started with an initial steroid therapy of 30 mg that was gradually decreased over 1 month, and the diplopia improved at 49 days after initiation. The headache disappeared on day 62 of the treatment, and he was in complete remission. Follow-up ASL was performed every other month showed reduced perfusion as the symptoms improved and confirmed the absence of a tumor over the follow-up period. However, the hotspot in the left cavernous sinus persisted even after the improvement in symptoms.
DISCUSSION
The diagnostic criteria for THS, updated in ICHD-3 in 2013, enabled THS to be diagnosed based on the presence of granulomatous inflammation on MRI, without confirmation by biopsy.[
Because THS lesions are small in size and located close to important structures such as the cavernous sinus, superior orbital fissure, or orbit, it is very difficult to obtain a pathological sample that demonstrates granulomatous inflammation. Biopsies are rarely performed, as in the present case. Coronal fast spin-echo T2WI and coronal fat-saturated T1WI with contrast have been shown effective for diagnosis.[
In the present patient, symptoms improved 2 months after onset. Serial imaging revealed that although ASL hyperperfusion gradually decreased, hyperperfusion remained even at the 3rd month after onset. Late hyperperfusion is suspected of residual and recurrence of granulomatous inflammation. Even if the symptoms improve, continued ASL perfusion MRI follow-up is essential to exclude tumors, vasculitis, skull base meningitis, and other diseases that cause painful ophthalmoplegia such as sarcoidosis and diabetes.[
Further case accumulation is required and reproducibility needs to be confirmed.
Steroid therapy is effective for treating THS. ASL can be performed repeatedly, without the use of contrast medium, to monitor therapeutic effect.[
CONCLUSION
ASL sequences performed routinely and repeatedly without contrast administration or ionizing radiation can aid in the diagnosis of THS. This simple technique will play an important role in confirming no recurrence after steroid therapy treatment.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Beppu T, Sato Y, Sasaki T, Terasaki K, Yamashita F, Sasaki M. Comparisons between PET With 11C-methyl-lmethionine and arterial spin labeling perfusion imaging in recurrent glioblastomas treated with bevacizumab. Clin Nucl Med. 2019. 44: 186-93
2. Cakirer S. MRI findings in Tolosa-Hunt syndrome before and after systemic corticosteroid therapy. Eur J Radiol. 2003. 45: 83-90
3. Cebeci H, Aydin O, Ozturk-Isik E, Inecikli F, Bekar A, Kocaeli H. Assesment of perfusion in glial tumors with arterial spin labeling; comparison with dynamic susceptibility contrast method. Eur J Radiol. 2014. 83: 1914-9
4. 5. Colnaghi S, Versino M, Marchioni E, Pichiecchio A, Bastianello S, Cosi V. ICHD-II diagnostic criteria for Tolosa-Hunt syndrome in idiopathic inflammatory syndromes of the orbit and/or the cavernous sinus. Cephalalgia. 2008. 28: 577-84 6. Hao R, He Y, Zhang H, Zhang W, Li X, Ke Y. The evaluation of ICHD-3 beta diagnostic criteria for Tolosa-Hunt syndrome: A study of 22 cases of Tolosa-Hunt syndrome. Neurol Sci. 2015. 36: 899-905 7. Kimura H, Takeuchi H, Koshimoto Y, Arishima H, Uematsu H, Kawamura Y. Perfusion imaging of meningioma by using continuous arterial spin-labeling: Comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol. 2006. 27: 85-93 8. Lai G, Mahadevan A, Hackney D, Warnke PC, Nigim F, Kasper E. Diagnostic accuracy of PET, SPECT, and arterial spin-labeling in differentiating tumor recurrence from necrosis in cerebral metastasis after stereotactic radiosurgery. AJNR Am J Neuroradiol. 2015. 36: 2250-5 9. Schuknecht B, Sturm V, Huisman TA, Landau K. Tolosa-Hunt syndrome: MR imaging features in 15 patients with 20 episodes of painful ophthalmoplegia. Eur J Radiol. 2009. 69: 445-53 10. Smith JL, Taxdal DS. Painful ophthalmoplegia. The Tolosa-Hunt syndrome. Am J Ophthalmol. 1966. 61: 1466-72 11. Zhang X, Zhou Z, Steiner TJ, Zhang W, Liu R, Dong Z. Validation of ICHD-3 beta diagnostic criteria for 13. 7 Tolosa-Hunt syndrome: Analysis of 77 cases of painful ophthalmoplegia. Cephalalgia. 2014. 34: 624-32