- Department of Neurosurgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
- Department of Neurosurgery, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
- Department of Surgery, College of Medicine, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Correspondence Address:
Wafa Faisal Aldhafeeri, Department of Neurosurgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_952_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Wafa Faisal Aldhafeeri1, Fehid Habalrih1, Ali Hamad Al Omar2, Atheer Abdulaziz Altamimi3, Marahib Saud Alshahrani1, Jamal Abdullah1, Abdulkarim Alrabie1, Syed Shah1. Postoperative cerebrospinal fluid infection by Ralstonia mannitolilytica: Two case reports and a literature review. 30-Dec-2022;13:602
How to cite this URL: Wafa Faisal Aldhafeeri1, Fehid Habalrih1, Ali Hamad Al Omar2, Atheer Abdulaziz Altamimi3, Marahib Saud Alshahrani1, Jamal Abdullah1, Abdulkarim Alrabie1, Syed Shah1. Postoperative cerebrospinal fluid infection by Ralstonia mannitolilytica: Two case reports and a literature review. 30-Dec-2022;13:602. Available from: https://surgicalneurologyint.com/surgicalint-articles/12081/
Abstract
Background: Ralstonia species are Gram-negative bacilli that are commonly found in moist environments, such as water and soil. They are opportunistic human pathogens, particularly found among immunocompromised patients, and are an infrequent cause of infection. The difficulty in correctly identifying and differentiating between Ralstonia species members using routine biochemical methods as well as their resistance to many classes of antibiotics poses a specific diagnostic and therapeutic challenge.
Case Description: We report two cases from our neurosurgical unit complicated by postoperative cerebrospinal fluid infection caused by Ralstonia Mannitolilytica that posed a therapeutic challenge.
Conclusion: Our hypothesis is contaminated irrigation fluids might be a significant cause of post-operative meningitis and prolonged hospital stay.
Keywords: Cerebrospinal fluid (CSF) infection, Meningitis, Ralstonia mannitolilytica
INTRODUCTION
Ralstonia mannitolilytica is an emerging opportunistic pathogen.[
We report two cases from our neurosurgical unit that was complicated by postoperative cerebrospinal fluid (CSF) infection caused by R. mannitolilytica.
CASE PRESENTATION
Case 1
A 9-year-old boy, who is a known case of epilepsy, was admitted electively to our neurosurgery unit as a case of suprasellar cystic lesion, for surgical resection. Our working diagnosis was craniopharyngioma [
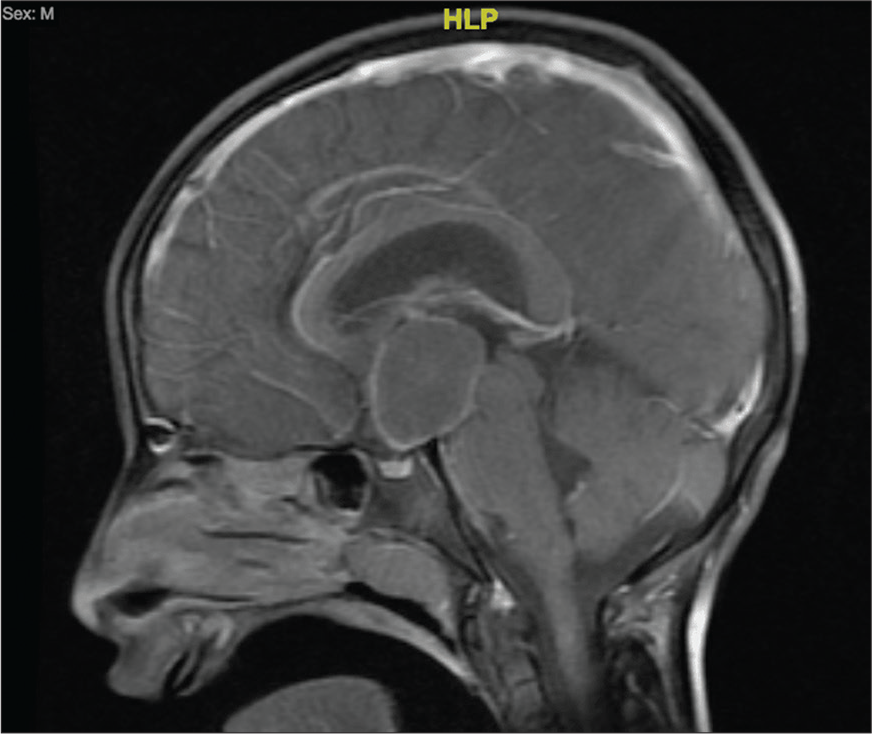
Figure 1:
Magnetic resonance imaging of the brain, T1-weighted gadolinium-enhanced. Sagittal view, showing hypothalamic/suprasellar cyst, measuring 2.2 × 2.3 × 3.2 cm in its maximum transverse, anteroposterior, and craniocaudal dimensions, respectively, with peripheral thin enhancement of the cyst and solid enhancement of the right inferolateral. HLP=head left posterior.
The patient underwent endoscopic cyst aspiration, biopsy, septostomy, and external ventricular drain (EVD) insertion. The surgery went well with no immediate postoperative complications. The patient was vitally stable, and he was transferred to pediatric intensive care unit for monitoring.
Day 1 postoperatively, the patient developed one spike of fever that responded to paracetamol. The patient was awake, conscious, and well-looking. His examination and basic laboratory investigations were within normal limits. The patient was on prophylactic cefazolin, as per our surgical prophylactic antibiotic protocol. Next day, the fever became more persistent. Septic workup was done, the patient was started on vancomycin and ceftazidime. The antibiotic regimen was modified to include gentamicin as the patient continued to have high grade fever. His inflammatory markers were within normal range. White blood cell count (WBC) was 12 × 109/l. Erythrocytes sedimentation rate (ESR) was 2 mm/h. C-reactive protein (CRP) was 1 mg/L. CSF examination taken from the EVD revealed WBC 392 cells/μL (74% polymorph), glucose 3.2 mmol/L, and protein 0.3 g/L. CSF culture resulted on day 5 postoperatively showing light growth of Gram-negative rods, namely, R. mannitolilytica. Other cultures were negative. The antibiotic regimen was down-graded to ceftazidime to complete 14 days. The EVD was removed and the tip was sent for culture. The same organism was isolated for EVD tip. Yet, the sensitivity report showed resistant development toward ceftazidime. Intravenous Ciprofloxacin was added to the regimen in light of the new sensitivity report. Antibiotics were stopped after clinical, laboratory, and radiological evidence of resolution.
Case 2
A 19-year-old female is known case of systemic lupus erythematosus and idiopathic intracranial hypertension (IIH). She underwent right ventriculoperitoneal (VP) Shunt insertion after she failed to respond to medical management. She was on prophylactic Cefazolin 25 mg/kg upon induction, and the same dose every 8 h 1 day postoperatively. The patient was discharged home with no immediate postoperative complications.
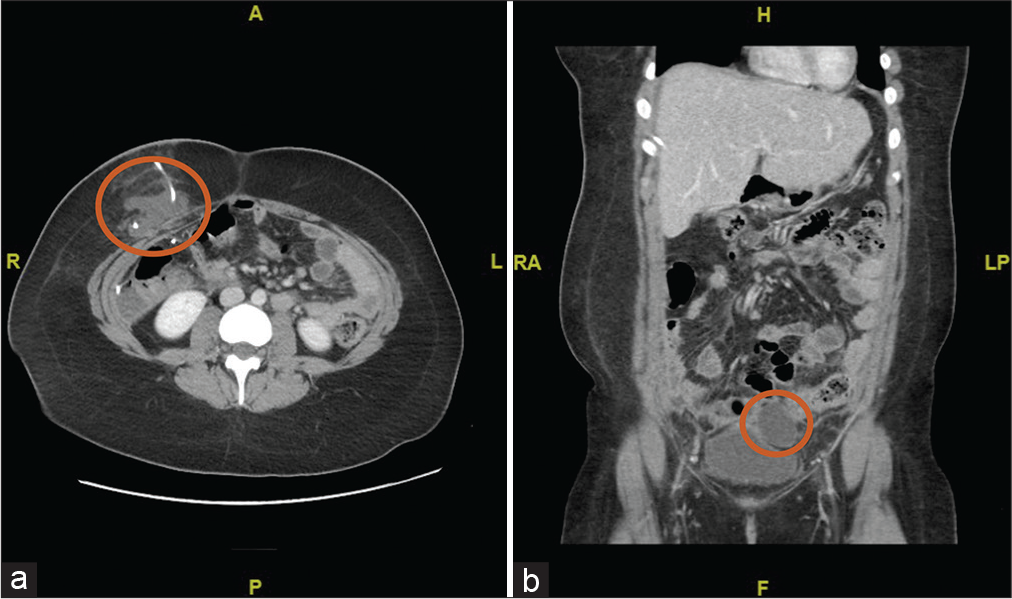
Two-week postoperatively, she presented to our emergency department with history of the right lower quadrant abdominal pain and high-grade fever for 3 days. On examination, she was febrile and tachycardic. Her Glasgow coma scale was 15/15. She had right lower quadrant tenderness. Her neurological examination was within normal limits, and no meningeal signs were observed. Septic screening was sent including CSF sample from shunt reservoir. Computed tomography (CT) of brain showed VP shunt in place and no collection. CT of the abdomen showed subcutaneous collection at VP shunt entry site measuring 3.7 × 6.0 cm with intrapelvic collection in the left supravesicular space measuring 4.1 × 5.3 cm [
Figure 2:
(a and b) Abdominal computed tomography image on (a) axial view Red circle: subcutaneous collection at shunt entry site (b) coronal view showing small subcutaneous collection at shunt entry site in the abdominal cavity measuring 3.7 × 6.0 cm, associated with small intrapelvic collection in the left supravesicular space causing minimal mass effect to the left lateral urinary bladder wall measuring 4.1 × 5.3 cm. RA= Right anterior / LP = left posterior / H=head/ F=feet, red circle: intrapelvic collection in the left supravesicular space causing minimal mass effect.
The patient was started empirically on Cefuroxime and Metronidazole. Yet the patient continued to have high-grade fever, so antibiotic was upgraded to Vancomycin and Ceftazidime. She underwent aspiration of abdominal collection and Jackson-Pratt drain was inserted, and fluids were sent for culture and sensitivity. Furthermore, VP shunt was removed, and both abdominal and cerebral tip were sent for culture. CSF culture resulted with heavy growth of R. mannitolilytica. Aspirated abdominal fluid culture showed no growth after 7 days. Pipracillin/tazobactam was started and other antibiotics were stopped. Next day, the patient continued to have high-grade fever. We started the patient on Ciprofloxacin and metronidazole. The patient started to improve clinically and repeated CT abdomen showed resolution of the abdominal collection. The patient was discharged later on oral antibiotics to complete 14 days. The patient declined further surgical treatment for IIH.
DISCUSSION
Bacteremia and postoperative CSF infections are associated with a high mortality and morbidity outcomes but data about systematic approach to the diagnosis, treatment, and management of R. mannitolilytica bloodstream and postoperative CSF infections are lacking.[
R. mannitolilytica has been described as a cause of recurrent ventricular-atrial shunt-associated meningitis.[
In spite of new antimicrobials and aseptic surgical techniques, postoperative meningitis (POM) results in considerable morbidity and mortality. Any neurosurgical intervention has a risk of developing POM with an incidence of 0.34–3.1%.[
CONCLUSION
We hypothesize that the infection was acquired following contamination by contaminated irrigation fluids during both procedures. Ralstonia species are not widely recognized as a major pathogen, despite their ability to induce serious multi-drug resistance infection. Clinician must consider them in differential diagnosis of postoperative CSF infections and sepsis to help in their early isolation and treatment.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Basso M, Venditti C, Raponi G, Navazio AS, Alessandri F, Giombini E. A case of persistent bacteraemia by Ralstonia mannitolilytica and Ralstonia pickettii in an intensive care unit. Infect Drug Resist. 2019. 12: 2391-5
2. Boattini M, Bianco G, Biancone L, Cavallo R, Costa C. Ralstonia mannitolilytica bacteraemia: A case report and literature review. Infez Med. 2018. 26: 374-8
3. Bonatti H. Ralstonia pickettii meningitis in a child with hydrocephalus. Eur J Pediatr Surg. 2009. 19: 341-2
4. Chitre G, Sirsath N. Ralstonia mannitolilytica outbreak in a day care oncology ward. J Patient Saf Infect Control. 2019. 7: 85-5
5. De Baere T, Steyaert S, Wauters G, De Vos P, Goris J, Coenye T. Classification of Ralstonia pickettii biovar 3/’ thomasii’ strains (pickett 1994) and of new isolates related to nosocomial recurrent meningitis as Ralstonia mannitolytica sp. nov. Int J Syst Evol Microbiol. 2001. 51: 547-58
6. Jhung MA, Sunenshine RH, Noble-Wang J, Coffin SE, St John K, Lewis FM. A national outbreak of Ralstonia mannitolilytica associated with use of a contaminated oxygen-delivery device among pediatric patients. Pediatrics. 2007. 119: 1061-8
7. Lim CT, Lee SE. A rare case of Ralstonia mannitolilytica infection in an end stage renal patient on maintenance dialysis during municipal water contamination. Pak J Med Sci. 2017. 133: 1047-9
8. Soavi L, Rosina M, Stefini R, Fratianni A, Cadeo B, Magri S. Post-neurosurgical meningitis: Management of cerebrospinal fluid drainage catheters influences the evolution of infection. Surg Neurol Int. 2016. 7: 927-34
9. Stelzmueller I, Biebl M, Wiesmayr S, Eller M, Hoeller E, Fille M. Ralstonia pickettiiinnocent by bystander or a potential threat?. Clin Microbiol Infect. 2006. 12: 99-101
10. Vaneechoutte M, De Baere T, Wauters G, Steyaert S, Claeys G, Vogelaers D. One case each of recurrent meningitis and hemoperitoneum infection with Ralstonia mannitolilytica. J Clin Microbiol. 2001. 39: 4588-90