- Department of Neurosurgery, Suwa Red Cross Hospital, Suwa, Nagano, Japan.
Correspondence Address:
Yukinari Kakizawa
Department of Neurosurgery, Suwa Red Cross Hospital, Suwa, Nagano, Japan.
DOI:10.25259/SNI_222_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki1, Yukinari Kakizawa1, Akihiro Nishikawa1, Yasunaga Yamamoto1, Toshiya Uchiyama1. Postsurgical functional outcome prediction model using deep learning framework (Prediction One, Sony Network Communications Inc.) for hypertensive intracerebral hemorrhage. 03-May-2021;12:203
How to cite this URL: Masahito Katsuki1, Yukinari Kakizawa1, Akihiro Nishikawa1, Yasunaga Yamamoto1, Toshiya Uchiyama1. Postsurgical functional outcome prediction model using deep learning framework (Prediction One, Sony Network Communications Inc.) for hypertensive intracerebral hemorrhage. 03-May-2021;12:203. Available from: https://surgicalneurologyint.com/surgicalint-articles/10781/
Abstract
Background: Reliable prediction models of intracerebral hemorrhage (ICH) outcomes are needed for decision-making of the treatment. Statistically making such prediction models needs a large number of samples and time-consuming statistical analysis. Deep learning (DL), one of the artificial intelligence, is attractive, but there were no reports on DL-based functional outcome prediction models for ICH outcomes after surgery. We herein made a functional outcome prediction model using DLframework, Prediction One (Sony Network Communications Inc., Tokyo, Japan), and compared it to original ICH score, ICH Grading Scale, and FUNC score.
Methods: We used 140 consecutive hypertensive ICH patients’ data in our hospital between 2012 and 2019. All patients were surgically treated. Modified Rankin Scale 0–3 at 6 months was defined as a favorable outcome. We randomly divided them into 100 patients training dataset and 40 patients validation dataset. Prediction One made the prediction model using the training dataset with 5-fold cross-validation. We calculated area under the curves (AUCs) regarding the outcome using the DL-based model, ICH score, ICH Grading Scale, and FUNC score. The AUCs were compared.
Results: The model made by Prediction One using 64 variables had AUC of 0.997 in the training dataset and that of 0.884 in the validation dataset. These AUCs were superior to those derived from ICH score, ICH Grading Scale, and FUNC score.
Conclusion: We easily and quickly made prediction models using Prediction One, even with a small single-center dataset. The accuracy of the DL-based model was superior to those of previous statistically calculated models.
Keywords: Artificial intelligence, Deep learning, Intracerebral hemorrhage, Machine learning, Prediction model
INTRODUCTION
Hypertensive intracerebral hemorrhage (ICH) is responsible for 10–30% of all strokes, and it is a significant cause of all stroke-related morbidity and mortality.[
Theoretically, surgical hematoma evacuation prevents herniation by reducing the intracranial pressure. It also decreases the pathophysiological impact of the ICH on surrounding tissue. However, the Surgical Trial in Intracerebral Hemorrhage (STICH) showed that patients with spontaneous supratentorial ICH showed no overall benefit from the early surgery when compared to the initial conservative therapy, though 24% of patients in the conservative group finally underwent surgery.[
We practice according to the Japanese Guidelines for the Management of Stroke 2009[
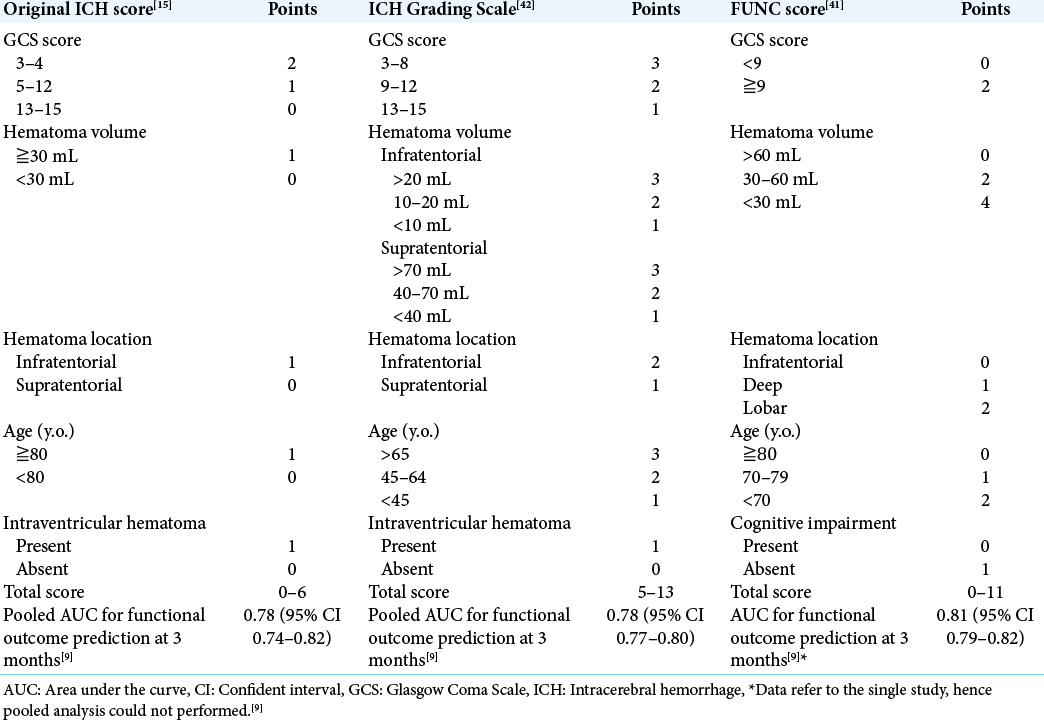
Previously, many studies tried to make the prediction model for ICH outcomes, including the original ICH score,[
Deep learning (DL), one of the machine learning, is recently attractive. DL is starting to be used in the neurosurgical situations in decision-making for spinal canal stenosis,[
We hypothesized that we could make a good prediction model for our own hospital using DL, even with a small dataset with detailed variables. Therefore, we herein produced the DL-based functional outcome prediction model using DL framework, Prediction One (Sony Network Communications Inc., Tokyo, Japan)[
MATERIALS AND METHODS
Study population
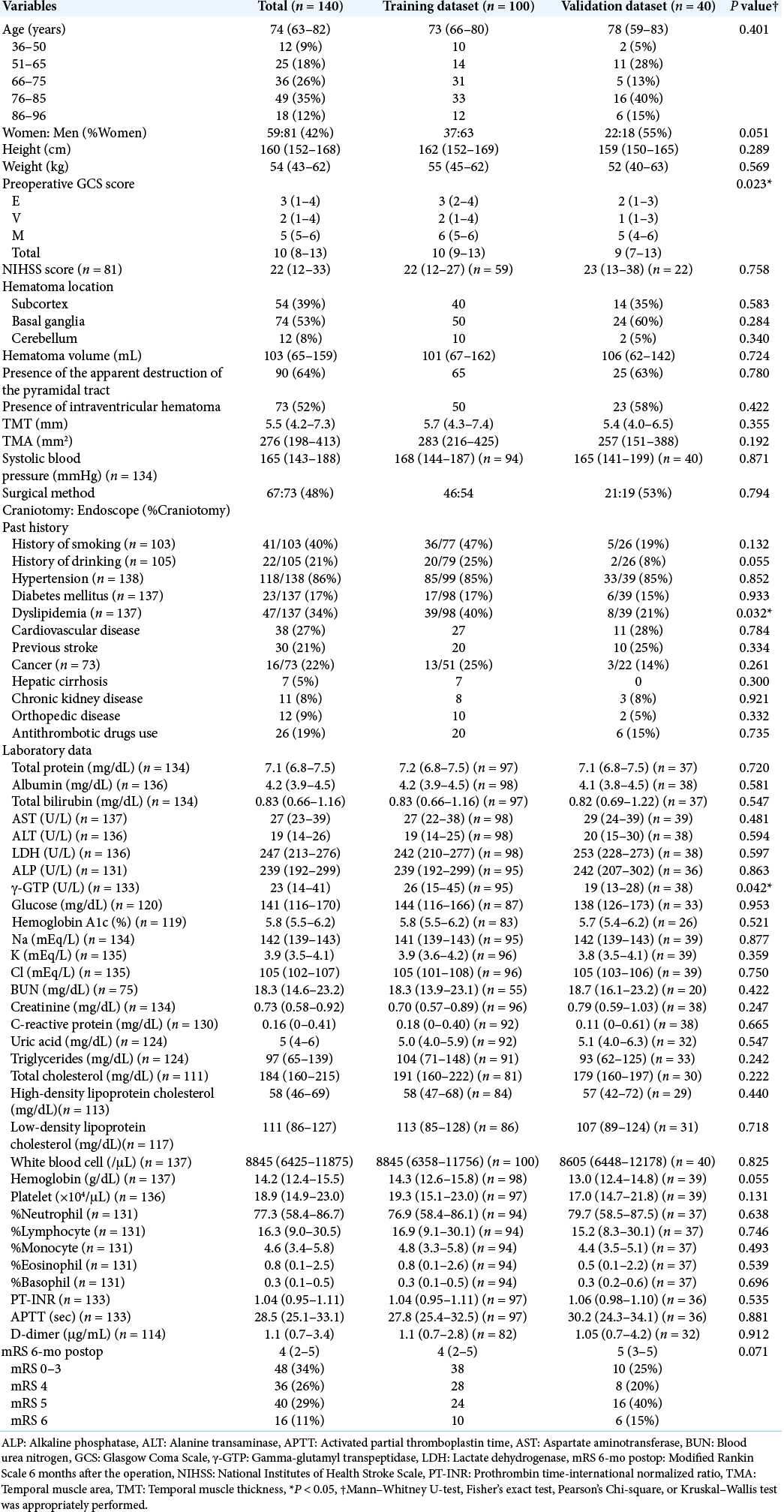
We retrospectively retrieved data from medical records of all the consecutive 140 hypertensive ICH patients admitted between 2012 and 2019 and surgically treated at our institution. Patients who did not undergo surgical treatment, those without GCS score at admission nor the outcome data at 6 months, were excluded from the study. The detail of the dataset is available online.[
General management
During admission and in the acute phase, patients were first administered nicardipine to maintain the normal systolic blood pressure at under 140 mmHg. The prothrombin time of patients undergoing anticoagulation therapy was normalized by the administration of Vitamin K and/or fresh frozen plasma. Then, a surgical indication was made following the Japanese Guidelines for the Management of Stroke 2009[
Hematoma removal with craniotomy was performed primarily from 2012 to 2013 and the endoscopic hematoma removal began in 2013. We gradually transitioned from craniotomy to endoscopic hematoma removal as a first-choice treatment between 2014 and 2015. During this period, patients who received antithrombotic drugs and displayed apparent extravasation on the contrast-enhanced CT image were likely to undergo a craniotomy. Since 2015, endoscopic procedures have been routinely performed in our hospital regardless of age, comorbidities, presence of antithrombotic drugs, and extravasation on the contrast-enhanced CT image. However, a craniotomy was still performed when the endoscope was unavailable due to reasons such as cleaning or the unavailability of the medical staff in the operating room (i.e., weekends and holidays). We performed craniotomy under general anesthesia but endoscopic hematoma removal under local anesthesia. The details of each surgical procedure and anesthesia method were described in our previous reports.[
Clinical variables
We collected data regarding physiological symptoms at admission for patients included in this study, that is, year, age, sex, height, weight, preoperative GCS score, National Institutes of Health Stroke Scale score, systolic blood pressure, administration of antithrombotic drugs, history of smoking and massive alcohol intake (over 450 g ethanol intake/week), and comorbidities (history or present treatment by a clinician for hypertension, diabetes mellitus, dyslipidemia, cardiovascular diseases, previous stroke, cancer, hepatic cirrhosis, chronic kidney diseases, or orthopedic disease). We also measured serum total protein, albumin, total bilirubin, aspartate aminotransferase, alanine transaminase, lactate dehydrogenase, alkaline phosphatase, gamma-glutamyl transpeptidase, glucose, hemoglobin A1c, sodium, potassium, chlorine, blood urea nitrogen, creatinine, C-reactive protein, uric acid, triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels. We also investigated whole and differential white blood cell counts, hemoglobin level, platelet count, prothrombin time-international normalized ratio, activated partial thromboplastin time, and D-dimer level.
We determined the location of the hematoma (subcortex, basal ganglia, or cerebellum) and measured hematoma volume by ABC/2 methods. We also checked the presence of intraventricular hematoma and the apparent destruction of the pyramidal tract. We observed the primary motor area, radiate corona, posterior limb of the internal capsule, and cerebral peduncle for potential destruction. The obvious destruction of these areas indicated that the pyramidal tract was apparently destroyed; while the equivocal one was absent. Moreover, the temporal muscle thickness and area[
We also investigated the treatment strategy (hematoma evacuation with craniotomy or endoscopic hematoma removal with or without neuronavigation). To evaluate the outcomes, modified Rankin Scale (mRS) scores at 6 months after the treatment of all 140 patients were collected by either personal outpatient interviews, reports from the rehabilitation hospital or home doctor, or interviews over the telephone, once the ethical approval was obtained for the study. We dichotomized mRS scores into favorable (mRS 0–3) or poor (mRS 4–6).
Making prediction model by prediction one
We used Prediction One framework to make the prediction model. We divided our 140 patients’ data randomly into 100 patients training dataset and 40 patient’s validation dataset. Prediction One read the 100 patients’ data with 64 variables, and automatically divided them into five-fold cross-validation datasets. Prediction One automatically adjusted and optimized the variables that are easy to be processed statistically and mathematically and select appropriate algorithms with ensemble learning. The missing values were automatically compensated, and Prediction One made the best prediction model by artificial neural network with internal five-fold cross-validation. The details are trade secrets and could not be provided.
We let the Prediction One framework make a functional outcome prediction model using 100 patients training dataset using 64 variables described above. The AUC of the model and strong variables with the weights were automatically calculated. Then, we performed the model’s validation using the 40 patients datasets, and calculated AUC, accuracy, precision, recall, and F value, which were used to evaluate the prediction model made by artificial intelligence.
Prediction using original ICH score, ICH Grading Scale, and FUNC score
We also investigated the original ICH score,[
We evaluated 3 scores’ AUCs calculated using the sum scores and the outcome, and compared them to AUCs of the model made by Prediction One.
Statistical analysis
Results are shown as median (interquartile range). The difference between the training dataset and the external validation dataset was tested using the Mann–Whitney U-test, Fisher’s exact test, Pearson’s Chi-square test, or Kruskal–Wallis test, appropriately. A two-tailed P < 0.05 was considered statistically significant. We calculated AUCs and their P values using SPSS software version 24.0.0 (IBM, New York, USA).
RESULTS
Clinical characteristics
The clinical characteristics of the 140 ICH patients (59 women and 81 men) are summarized in [
Model development and validation
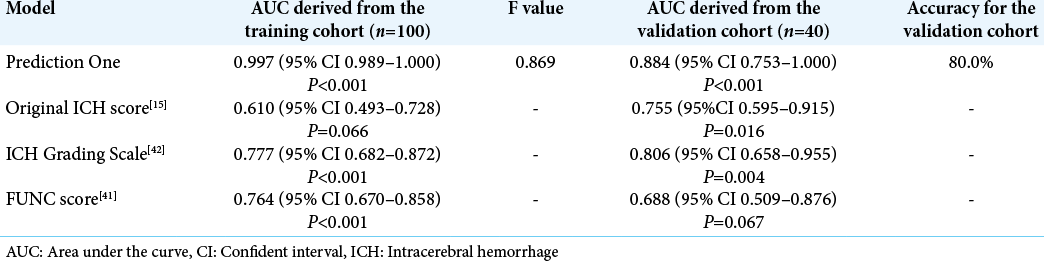
Prediction One produced the functional outcome prediction model using the 100 patients with 64 variables in <4 min. The AUC of the model was 0.997 (95% confident interval [CI] 0.989–1.000). The model’s accuracy, precision, recall, and F value were 0.810, 1.000, 0.769, and 0.869, respectively. Its AUC for the validation dataset were 0.884 (95% CI 0.753– 1.000) with 80.0% accuracy [
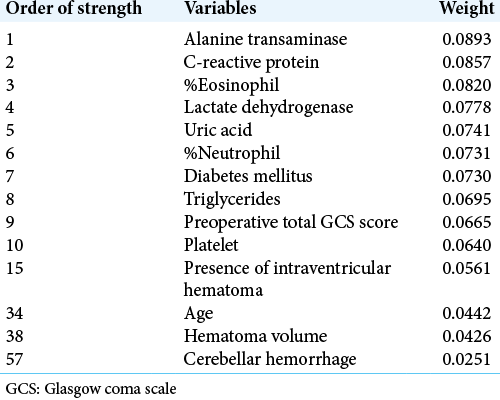
The stronger variables and their weights of the model are listed in [
Comparison to original ICH score, ICH Grading Scale, and FUNC score
We calculated original ICH score, ICH Grading Scale, and FUNC score in the training and validation dataset, respectively. The AUCs of these scores in the training dataset were 0.610 (95% CI 0.493–0.728), 0.777 (95% CI 0682–872), and 0.764 (95% CI 0.670–0.858), respectively. Those in the validation dataset were 0.755 (95% CI 0.595–0.915), 0.806 (95% CI 0.658–0.955), and 0.688 (95% CI 0.509–0.876), respectively. These AUCs were all inferior to the models made by Prediction One [
DISCUSSION
We made the postsurgical functional outcome prediction model using the DL framework, Prediction One. We created the model with a high prediction rate using a small dataset (n = 100) with several missing data. It would be reliable for the functional prediction in our own hospital with 80% accuracy. Furthermore, this is the first report on creating a functional outcome prediction model of postoperative ICH patients using DL.
Advantages of DL
Conventional time and cost-consuming statistical analysis need laborious standardization of variables like a logarithmic transformation to increase the prediction model’s accuracy. It also requires the arbitrary selection of variables based on the previous studies, and multivariate analysis needs 10 folds number of samples against the variables.[
We then review these benefits of DL in our study. Conventionally, we could have used only ten variables for statistical analysis due to the small sample size of the training dataset (n = 100). Furthermore, the dataset contains several missing data. However, we could use 64 variables for making the prediction model by Prediction One, and make a good prediction model from the small dataset. We did not need to perform variable optimization nor manipulations for the missing values. Furthermore, some unexpected serological test results such as alanine transaminase, C-reactive protein, %eosinophil, lactate dehydrogenase, and uric acid levels were judged to be more important among many other previously reported important factors, such as age, hematoma volume, and hematoma location. We usually think that some variables, such as age, hematoma volume, and preoperative GCS, largely affect the outcomes, with common sense. However, the DL framework treats many variables equally and without preconceptions, revealing the very important factors that were not expected.
Besides, the time needed for creating each model was <4 min. Finally, the models achieved high accuracy with the AUC of 0.997 in the training dataset and that of 0.884 in the validation dataset. Putting it bluntly, our study showed that our DL-based prediction model, even made from the small dataset, can predict the ICH patients’ outcomes surgically treated in our hospital with higher accuracy than other scores, which was made from the large cohort study.
Recent study on artificial intelligence and ICH
Andrew reported that decision tree and random forests could be useful to predict 3 months functional outcomes.[
Our study is the first attempt to make a prediction model using DL, not other machine learning methods. DL treats variables comprehensively, so it could not present particularly important factors as in a decision tree or XGBoost, and Prediction One could not treat CT radiomics. However, the AUC of the model in the training dataset of 0.997 and that in the validation dataset 0.884 were much higher compared to the other machine learning methods in these previous studies. It is also a strong point that we could easily create a prediction model in <4 min without vigorous effort except for collecting data. Each artificial intelligence algorithm has its own merits and demerits, and it is necessary to consider which method is better in the future.
Future outlook
Despite the easiness, advantages, and future potential of DL, the majority of medical staff cannot treat DL frameworks.[
Limitation of this study
First, we did not use other scores, such as Essen-ICH score,[
CONCLUSION
We easily and quickly made the functional outcome prediction model using Prediction One framework, and it is superior to other prediction scores, such as the original ICH score, ICH Grading Scale, and FUNC score, which were statistically calculated with a large cohort. Even with a small single-center dataset, containing missing data, prognostic models made by the DL framework can be useful at the institution and may be beneficial for us to determine the surgical indication.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Azimi P, Mohammadi HR, Benzel EC, Shahzadi S, Azhari S. Use of artificial neural networks to decision making in patients with lumbar spinal canal stenosis. J Neurosurg Sci. 2017. 61: 603-11
2. Cho DY, Chen CC, Lee WY, Lee HC, Ho LH. A new Modified Intracerebral Hemorrhage score for treatment decisions in basal ganglia hemorrhage--a randomized trial. Crit Care Med. 2008. 36: 2151-6
3. Chuang YC, Chen YM, Peng SK, Peng SY. Risk stratification for predicting 30-day mortality of intracerebral hemorrhage. Int J Qual Health Care. 2009. 21: 441-7
4. Fukuma R, Yanagisawa T, Kinoshita M, Shinozaki T, Arita H, Kawaguchi A. Prediction of IDH and TERT promoter mutations in low-grade glioma from magnetic resonance images using a convolutional neural network. Sci Rep. 2019. 9: 20311
5. Furtner J, Berghoff AS, Albtoush OM, Woitek R, Asenbaum U, Prayer D. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur Radiol. 2017. 27: 3167-73
6. Furtner J, Berghoff AS, Schöpf V, Reumann R, Pascher B, Woitek R. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J Neurooncol. 2018. 140: 173-8
7. Furtner J, Genbrugge E, Gorlia T, Bendszus M, Nowosielski M, Golfinopoulos V. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019. 21: 1587-94
8. Fuse N, Sakurai-Yageta M, Katsuoka F, Danjoh I, Shimizu R, Tamiya G. Establishment of integrated biobank for precision medicine and personalized healthcare: The tohoku medical megabank project. JMA J. 2019. 2: 113-22
9. Gregório T, Pipa S, Cavaleiro P, Atanásio G, Albuquerque I, Chaves PC. Assessment and comparison of the four most extensively validated prognostic scales for intracerebral hemorrhage: Systematic review with meta-analysis. Neurocrit Care. 2019. 30: 449-66
10. Gregson BA, Mitchell P, Mendelow AD. Surgical decision making in brain hemorrhage. Stroke. 2019. 50: 1108-15
11. Gupta VP, Garton ALA, Sisti JA, Christophe BR, Lord AS, Lewis AK. Prognosticating functional outcome after intracerebral hemorrhage: The ICHOP score. World Neurosurg. 2017. 101: 577-83
12. Hall AN, Weaver B, Liotta E, Maas MB, Faigle R, Mroczek DK. Identifying modifiable predictors of patient outcomes after intracerebral hemorrhage with machine learning. Neurocrit Care. 2021. 34: 73-84
13. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019. 393: 1021-32
14. Hasegawa Y, Yoshida M, Sato A, Fujimoto Y, Minematsu T, Sugama J. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr Gerontol Int. 2019. 19: 135-40
15. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001. 32: 891-7
16. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2015. 46: 2032-60
17. Ido K, Nakamura N, Nakayama M. Miyagi medical and welfare information network: A backup system for patient clinical information after the great East Japan Earthquake and Tsunami. Tohoku J Exp Med. 2019. 248: 19-25
18. Iihara K, Tominaga T, Saito N, Suzuki M, Date I, Fujii Y. The Japan neurosurgical database: Overview and results of the first-year survey. Neurol Med Chir (Tokyo). 2020. 60: 165-90
19. Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y. A novel risk score to predict 1-year functional outcome after intracerebral hemorrhage and comparison with existing scores. Crit Care. 2013. 17: R275
20. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011. 56: 34-40
21. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. Easily created prediction model using deep learning software (Prediction One, Sony Network Communications Inc) for subarachnoid hemorrhage outcomes from small dataset at admission. Surg Neurol Int. 2020. 11: 374
22. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. Lower total protein and absence of neuronavigation are novel poor prognostic factors of endoscopic hematoma removal for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020. 29: 105050
23. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. The dataset on the characteristics of the intracerebral hemorrhage patients treated by endoscopic hematoma removal or craniotomy. Data Brief. 2020. 33: 106387
24. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. Endoscopic hematoma removal of supratentorial intracerebral hemorrhage under local anesthesia reduces operative time compared to craniotomy. Sci Rep. 2020. 10: 10389
25. Katsuki M, Narita N, Ishida N, Watanabe O, Cai S, Ozaki D. Preliminary development of a prediction model for daily stroke occurrences based on meteorological and calendar information using deep learning framework (Prediction One; Sony Network Communications Inc. Japan). Surg Neurol Int. 2021. 12: 31
26. Katsuki M, Narita N, Matsumori Y, Ishida N, Watanabe O, Cai S. Preliminary development of a deep learning-based automated primary headache diagnosis model using Japanese natural language processing of medical questionnaire. Surg Neurol Int. 2020. 11: 475
27. Katsuki M, Suzuki Y, Kunitoki K, Sato Y, Sasaki K, Mashiyama S. Temporal muscle as an indicator of sarcopenia is independently associated with hunt and kosnik grade on admission and the modified rankin scale score at 6 months of patients with subarachnoid hemorrhage treated by endovascular coiling. World Neurosurg. 2020. 137: e526-34
28. Katsuki M, Suzuki Y, Kunitoki K, Sato Y, Sasaki K, Mashiyama S. Temporal muscle thickness and area with various characteristics data of the patients with aneurysmal subarachnoid hemorrhage who underwent endovascular coiling. Data Brief. 2020. 31: 105715
29. Katsuki M, Yamamoto Y, Uchiyama T, Nishikawa A, Wada N, Kakizawa Y. Temporal muscle thickness and area with various characteristics data of the elderly patients over 75 with aneurysmal subarachnoid haemorrhage whose World Federation of Neurosurgical Societies grade were I to III. Data Brief. 2020. 28: 104832
30. Katsuki M, Yamamoto Y, Uchiyama T, Wada N, Kakizawa Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia?. Clin Neurol Neurosurg. 2019. 186: 105535
31. Kellner CP, Song R, Pan J, Nistal DA, Scaggiante J, Chartrain AG. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. 2020. 12: 489-94
32. Kobayashi Y.editors. Japanese Stroke Databank 2015. Tokyo: Nakayama Shoten; 2015. p.
33. Kumar R, Gupta A, Arora HS, Pandian GN, Raman B. CGHF: A computational decision support system for glioma classification using hybrid radiomics-and stationary wavelet-based features. IEEE Access. 2020. 8: 79440-58
34. Lao J, Chen Y, Li ZC, Li Q, Zhang J, Liu J. A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep. 2017. 7: 10353
35. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013. 382: 397-408
36. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005. 365: 387-97
37. Nie X, Cai Y, Liu J, Liu X, Zhao J, Yang Z. Mortality prediction in cerebral hemorrhage patients using machine learning algorithms in intensive care units. Front Neurol. 2020. 11: 610531
38. Onodera H, Mogamiya T, Matsushima S, Sase T, Kawaguchi K, Nakamura H. High protein intake after subarachnoid hemorrhage improves oral intake and temporal muscle volume. Clin Nutr. 2021. 40: 495
39. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996. 49: 1373-9
40. Ranganathan K, Terjimanian M, Lisiecki J, Rinkinen J, Mukkamala A, Brownley C. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J Surg Res. 2014. 186: 246-52
41. Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008. 39: 2304-9
42. Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martínez JJ, González-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke. 2007. 38: 1641-4
43. Sembill JA, Gerner ST, Volbers B, Bobinger T, Lücking H, Kloska SP. Severity assessment in maximally treated ICH patients: The max-ICH score. Neurology. 2017. 89: 423-31
44. Prediction One. Available from: https://www.predictionone.sony.biz. [Last accessed on 2020 Feb 29].
45. Staartjes VE, Stumpo V, Kernbach JM, Klukowska AM, Gadjradj PS, Schröder ML. Machine learning in neurosurgery: A global survey. Acta Neurochir (Wien). 2020. 162: 3081-91
46. Steindl A, Leitner J, Schwarz M, Nenning KH, Asenbaum U, Mayer S. Sarcopenia in neurological patients: Standard values for temporal muscle thickness and muscle strength evaluation. J Clin Med. 2020. 9: 1272
47. 48. 49. UPenn and Mayo Clinic’s Seizure Detection Challenge Detect Seizures in Intracranial EEG Recordings. Available from: https://www.kaggle.com/c/seizure-detection. [Last accessed on 2020 Dec 28]. 50. Vespa P, Hanley D, Betz J, Hoffer A, Engh J, Carter R. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage: A multicenter randomized controlled trial. Stroke. 2016. 47: 2749-55 51. Watanabe O, Narita N, Katsuki M, Ishida N, Cai S, Otomo H. Prediction model of deep learning for ambulance transports in kesennuma city by meteorological data. Open Access Emerg Med. 2021. 13: 23-32 52. Weimar C, Benemann J, Diener HC. Development and validation of the essen intracerebral haemorrhage score. J Neurol Neurosurg Psychiatry. 2006. 77: 601-5 53. Xu X, Zhang J, Yang K, Wang Q, Chen X, Xu B. Prognostic prediction of hypertensive intracerebral hemorrhage using CT radiomics and machine learning. Brain Behav. 2021. 2021: e02085