- Department of Neurosurgery, Diponegoro University, Semarang, Central Java, Indonesia.
- Department of Neurosurgery, Faculty of Medicine, Diponegoro University, Semarang, Central Java, Indonesia.
Correspondence Address:
Yuriz Bakhtiar, Department of Neurosurgery, Diponegoro University, Semarang, Central Java, Indonesia.
DOI:10.25259/SNI_142_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuriz Bakhtiar1, Novita Ikbar Khairunnisa1, Krisna Tsaniadi Prihastomo1, Happy Kurnia Brotoarianto2, Muhamad Thohar Arifin1, Zainal Muttaqin1. Posttraumatic epilepsy: A single institution case series in Indonesia. 15-Jul-2022;13:298
How to cite this URL: Yuriz Bakhtiar1, Novita Ikbar Khairunnisa1, Krisna Tsaniadi Prihastomo1, Happy Kurnia Brotoarianto2, Muhamad Thohar Arifin1, Zainal Muttaqin1. Posttraumatic epilepsy: A single institution case series in Indonesia. 15-Jul-2022;13:298. Available from: https://surgicalneurologyint.com/surgicalint-articles/11725/
Abstract
Background: Posttraumatic epilepsy (PTE) is a debilitating sequelae following traumatic brain injury (TBI). Risk of developing PTE is higher in the first 6 months following head trauma and remains increased for 10 years. Many cases of PTE developed into drug-resistant epilepsy in which need surgical treatment.
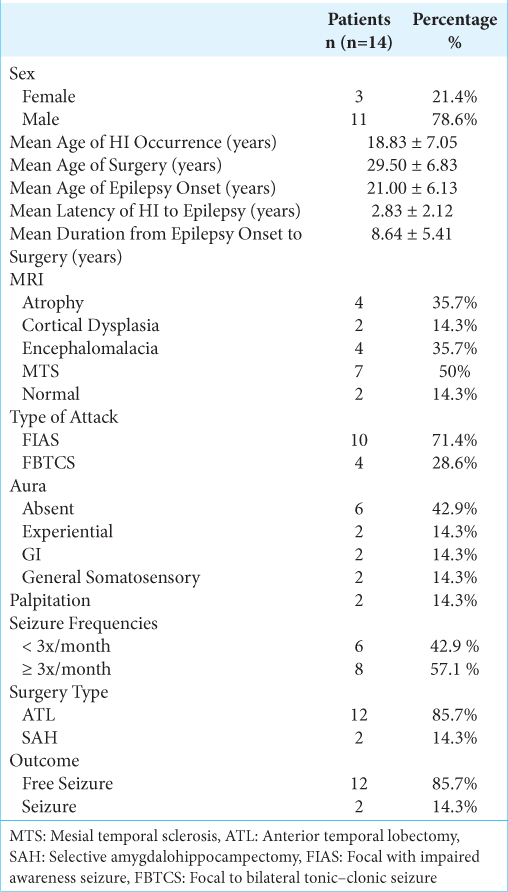
Case Description: Fourteen patients were identified from 1998 until 2021. Mean age at onset was 21.00 ± 6.13 years, mean age of surgery was 29.50 ± 6.83 years. All patients had partial complex seizure with more than half of cases (n = 10, 71.4%) reported with focal impaired awareness seizure and focal to bilateral tonic–clonic type of seizure which were observed in the remained cases (n = 4, 28.6%). Abnormal magnetic resonance imaging findings were observed in 12 patients: mesial temporal sclerosis (n = 7), encephalomalacia (n = 4), brain atrophy (n = 4), and focal cortical dysplasia (n = 2). More than half of cases presented with mesial temporal lobe epilepsy despite site and type of brain injury. Most patients who undergone epileptogenic focus resection were free of seizure, but two patients remained to have seizure with worthwhile improvement.
Conclusion: This study emphasizes the clinical characteristic of PTE cases in our center in Indonesia. While encephalomalacia is a typical finding following TBI and often responsible for epilepsy, electroencephalogram recording remains critical in determining epileptic focus. Most of PTE patients presented with temporal lobe epilepsy had excellent outcomes after surgical resection of epileptogenic focus.
Keywords: Epilepsy, Posttraumatic epilepsy, Seizure, Temporal lobe epilepsy, Traumatic brain injury
INTRODUCTION
Approximately 69 million people worldwide each year are affected by traumatic brain injury (TBI). The highest incidences have been reported from the United States and Europe with 1299 cases/100,000 people and 1012 cases/100,000 people, respectively.[
Posttraumatic epilepsy (PTE) is disabling sequelae of TBI that accounts for 20% of all acquired epilepsy and is frequently drug resistant. The risk to developing PTE is higher in patients who have suffered severe brain injury with structural damage.[
Reports of PTE are scarce in low- and middle-income countries, including Indonesia. Herein, we present a case series of medically intractable PTE patients who were eligible for surgical treatment from 1998 to 2021.
METHODS
This is a retrospective and Institutional Review Board approved study. We use our admission database to identified posttraumatic epilepsy patients from 1998 to 2021. Patients who admitted for epileptic surgery with documented history of TBI (skull fracture; intracerebral or intracranial hemorrhage; or traumatic encephalomalacia on neuroimaging obtained nonacutely during their epilepsy) were selected in this study. Records were reviewed for demographic information, magnetic resonance imaging (MRI) findings, ictal electroencephalogram (EEG) recording, and type of surgery. Engel class classification was used to assess patient outcomes at least 1 year following epileptic focus surgical procedure. Informed consent was obtained from the patients.
ILLUSTRATIVE CASE
Case 1
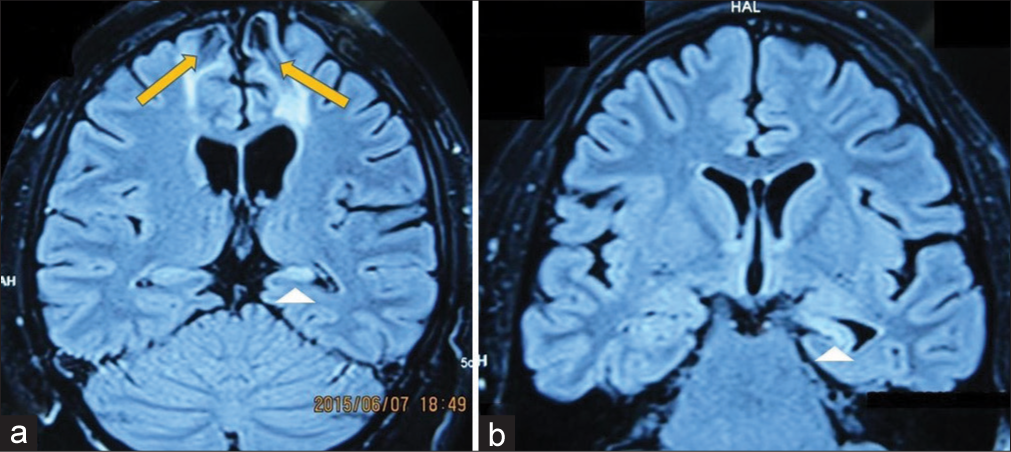
A 24-year-old male who suffered from drug-resistant epilepsy was admitted to our hospital. His medical history was consistent with bilateral frontal contusion resulted from vehicle collision. Three years after the accident, a focal impaired awareness seizure was observed. The seizure begins with sudden uncomfortable sensation in the head, followed by uncontrolled movement of the eyes to the right, dystonia of the right arm, and loss of consciousness. He had received two antiepileptic drugs: carbamazepine and lamotrigine but free seizure state was not accomplished. MRI revealed bilateral frontal encephalomalacia and left mesial temporal sclerosis (MTS) (
CASE DESCRIPTION
Fourteen posttraumatic epilepsy patients were enrolled in this study. Eleven (78.6%) patients were male, mean age of surgery was 29.50 ± 6.83 years, mean age at epilepsy onset 21.00 ± 6.13 years, and mean latency from head injury to epilepsy onset 2.83 ± 2.12 years [
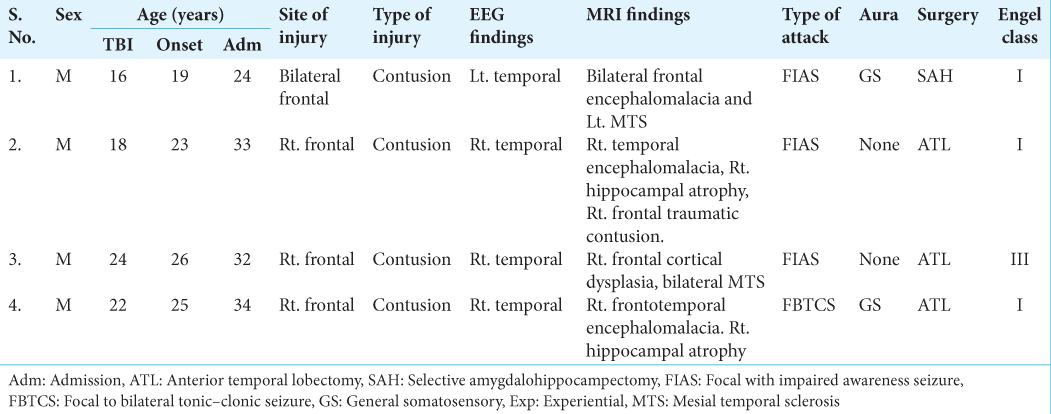
We present four cases of encephalomalacia following TBI who presented with temporal lobe epilepsy in
DISCUSSION
PTE accounts for 20% of acquired epilepsy, with severe brain injuries increasing the incidence to 16% over a 30-year period.[
Seizure after TBI is classified into immediate (occurring within 24 h), early (within the first 7 days), and late (occurring after 7 days).[
A study by Xu et al. identifies PTE-related risk factors: sex, history of alcohol abuse, focal neurological sign, posttraumatic amnesia, and loss of consciousness following TBI. Abnormal imaging findings in TBI such as skull fracture, midline shifting, contusions, intracranial hemorrhage, and subdural hemorrhage also pose a higher risk for the occurrence of PTE.[
Encephalomalacia is a common lesion resulting from TBI and often responsible for epilepsy. Recent study by Wang et al. revealed that FLAIR hyperintense part surrounding encephalomalacia contains more dendrites. Higher neural density was thought to be correlated with the production of repetitive excitatory circuit which leads to epilepsy. Larger encephalomalacia and larger hyperintense lesion were also common findings in epilepsy group.[
Following a TBI, the brain initiates acute neuronal and glial responses that often result in considerable cell loss in lesional and perilesional areas and long-term changes in the architecture of neural networks, most notably in the hippocampus and neocortex. Within minutes to hours following injury, shearing of white matter tracts, contusions, hematomas, and edema result in neurotransmitter release, free radical generation, calcium-mediated damage, angiogenesis, mitochondrial dysfunction, and inflammatory responses, all of which have been linked to epileptogenesis.[
Direct injury to cortical drivers after TBI triggers pathogenesis in hippocampus and is associated with both limbic seizures and thalamocortical like seizures that present different clinical correlates depending on the ictal sites.[
Temporal lobe epilepsy is the most commonly reported subtype in series of PTE. Histopathologic studies in PTE patients have demonstrated that diffuse temporal neocortical and hippocampal cell loss are present in most cases undergoing surgery.[
This study has several limitations including small sample size and the retrospective nature. Due to its small sample size, determining the statistical significance was challenging. In addition, retrospective study is subject to bias of unmeasured factors. Our study may not accurately reflect the true number of PTE cases as we omitted drug-sensitive epilepsy in this study. Therefore, multicenter study that incorporates both drug-resistant and drug-sensitive PTE is needed to reflect the true number of PTE in Indonesia, which we believe to be quite high.
CONCLUSION
This study emphasizes the clinical characteristic of PTE cases in our center in Indonesia. While encephalomalacia is a typical finding following TBI and often responsible for epilepsy, electroencephalogram recording remains critical in determining epileptic focus. Most of PTE patients presented with temporal lobe epilepsy had excellent outcomes after surgical resection of epileptogenic focus. Clinician should be careful to determine the region of interest in the setting of multiple structural abnormalities. Therefore, comprehensive and throughout examinations are mandatory in epilepsy cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: An overview. Clin Neurol Neurosurg. 2006. 108: 433-9
2. D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005. 128: 174-88
3. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018. 130: 1080-97
4. Frey LC. Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia. 2003. 44: 11-7
5. Gupta PK, Sayed N, Ding K, Agostini MA, Van Ness PC, Yablon S. Subtypes of post-traumatic epilepsy: Clinical, electrophysiological, and imaging features. J Neurotrauma. 2014. 31: 1439-43
6. Hakimian S, Kershenovich A, Miller JW, Ojemann JG, Hebb AO, D’Ambrosio R. Long-term outcome of extratemporal resection in posttraumatic epilepsy. Neurosurg Focus. 2012. 32: E10
7. Hitti FL, Piazza M, Sinha S, Kvint S, Hudgins E, Baltuch G. Surgical outcomes in post-traumatic epilepsy: A single institutional experience. Oper Neurosurg (Hagerstown). 2020. 18: 12-8
8. Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D. Commonalities in epileptogenic processes from different acute brain insults: Do they translate?. Epilepsia. 2018. 59: 37-66
9. Pitkänen A, Kyyriäinen J, Andrade P, Pasanen L, Ndode-Ekane XE.editors. Epilepsy after traumatic brain injury. Models Seizures Epilepsy. Cambridge, Massachusetts: Academic Press; 2017. p. 661-81
10. Rao VR, Parko KL. Clinical approach to posttraumatic epilepsy. Semin Neurol. 2015. 35: 57-63
11. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017. 58: 512-21
12. Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006. 47: 1373-82
13. Wang D, Shang K, Sun Z, Li YH. Experimental Imaging study of encephalomalacia fluid-attenuated inversion recovery (FLAIR) hyperintense lesions in posttraumatic epilepsy. Neural Plast. 2021. 2021: 2678379
14. Willmore LJ, Ueda Y. Posttraumatic epilepsy: Hemorrhage, free radicals and the molecular regulation of glutamate. Neurochem Res. 2009. 34: 688-97
15. Xu T, Yu X, Ou S, Liu X, Yuan J, Huang H. Risk factors for posttraumatic epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2017. 67: 1-6