- Department of Neurosurgery, Zagazig University, Zagazig, Alsharkia, Egypt.
- Department of Anesthesia and Surgical Intensive Care, Zagazig University, Zagazig, Alsharkia, Egypt.
- Department of Neurosurgery, Tanta University, Tanta, Algharbiya, Egypt.
Correspondence Address:
Ahmed A. Morsy
Department of Neurosurgery, Tanta University, Tanta, Algharbiya, Egypt.
DOI:10.25259/SNI_873_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed A. Morsy1, Ayman M. Ismail1, Yasser M. Nasr2, Salwa H. Waly2, Esam A. Abdelhameed3. Predictors of stimulation-induced seizures during perirolandic glioma resection using intraoperative mapping techniques. 24-Mar-2021;12:117
How to cite this URL: Ahmed A. Morsy1, Ayman M. Ismail1, Yasser M. Nasr2, Salwa H. Waly2, Esam A. Abdelhameed3. Predictors of stimulation-induced seizures during perirolandic glioma resection using intraoperative mapping techniques. 24-Mar-2021;12:117. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10663
Abstract
Background: Intraoperative mapping techniques maximize safety and efficacy during perirolandic glioma resection but may induce seizures and limit the procedure. We aim to report the incidence and predictors of stimulation-induced seizures during mapping either patient is awake or under general anesthesia (GA).
Methods: Retrospective analysis of 64 patients (40 awake and 24 GA) with perirolandic glioma underwent resection using intraoperative mapping techniques between 2014 and 2019. Preoperative data, operative details, postoperative neurological status, and extent of resection (EOR) were analyzed. Predictors of intraoperative seizures were assessed.
Results: The mean cortical and subcortical stimulation intensities needed to evoke motor responses were significantly lower in awake cases than in GA patients (4.9 ± 0.42 vs. 8.9 ± 1.2 mA) and (8.3 ± 0.62 vs. 12.1 ± 1.1 mA), respectively (P = 0.01). Incidence of intraoperative seizures was lower but statistically non-significant in awake cases (10% vs. 12.5%) (P = 0.76). Preoperative multiple antiepileptic drugs (AEDs) (P = 0.03) and low-grade glioma (P = 0.04) were statistically significant predictors for intraoperative seizures. Mean EOR in awake cases was 92.03% and 90.05% in GA cases (P = 0.23). Postoperative deficits were permanent after 3 months only in 5% of awake patients versus 8.3% of GA group (P = 0.59).
Conclusion: Awake craniotomy with intraoperative mapping can be done safely for perirolandic gliomas with lower but statistically nonsignificant incidence of intraoperative seizures and this could be attributed to statistically significant lower stimulation intensities required for mapping. Preoperative multiple AEDs and low-grade glioma are significant predictors for intraoperative seizures.
Keywords: Awake craniotomy, Brain mapping, Eloquent areas, Intraoperative seizures, Perirolandic glioma
INTRODUCTION
Surgical resection of perirolandic gliomas within or adjacent to sensorimotor eloquent areas represents a great challenge for neurosurgeons.[
Intraoperative mapping techniques including cortical and subcortical stimulation can be done to identify motor pathways during perirolandic glioma resection, while patient is awake to utilize patient’s cooperation in continuous assessment of voluntary motor movements during resection, or patient is under general anesthesia (GA).[
Recent studies recommended intraoperative mapping, while patient is awake for better neurological outcomes and higher EOR compared to similar surgery done under GA.[
In this study, we aim to report the incidence and evaluate predictors of intraoperative stimulation-induced seizures during perirolandic glioma resection using mapping techniques either awake or under GA, as one of the most important intraoperative risks which can affect the surgical outcomes. EOR and postoperative neurological outcome were also analyzed to evaluate role of intraoperative mapping in such cases.
MATERIALS AND METHODS
Patients population
This retrospective study was based on collected data of 64 patients with hemispheric perirolandic glioma (3 cm anterior or posterior to the motor cortex) who underwent surgical resection using intraoperative cortical and subcortical mapping techniques either under awake craniotomy (40 patients) or craniotomy under GA (24 patients), between 2014 and 2019 by the same neurosurgery and anesthesia teams. The institutional review board approved this study.
Patients with the following inclusion criteria were operated under awake craniotomy (awake group): (1) Age >18 and <70 years old without major cardiopulmonary co-morbidities. (2) Fluent in speaking and understanding without preoperative cognitive impairment (mini mental state examination more than 24). (3) Do not have severe language deficits (greater than 30% of naming errors) or motor deficits less than antigravity motor function. (4) Do not show severe anxiety or emotional instability (The State-Trait Anxiety Inventory score less than 55).[
Patients, who did not meet these criteria, were operated under (GA Group) using total intravenous protocol (TIVA), with intraoperative neurophysiological mapping and monitoring techniques. Written informed consent was also obtained from all patients.
Patients’ demographics, co-morbidities, presenting symptoms, preoperative seizure history and medications, preoperative neurological examination, operative details including intraoperative electrophysiological mapping and monitoring values, immediate and late postoperative neurological status, histopathology, and EOR based on volumetric analysis of pre- and postoperative MRI studies were collected. The Karnofsky Performance Scale (KPS) was used to assess preoperative and postoperative functional status.
Preoperative evaluation
Preoperative full physical and neuropsychological assessments were done for all patients and type of procedure either awake or under GA was chosen by the senior surgeon according to the inclusion criteria mentioned before. For all patients, MRI brain with contrast was done to determine tumor location, characters, and size, then fMRI and DTI were done to detect relation of the tumor to the cortical and subcortical motor tracts. In our cases, the use of anticonvulsant drugs was necessary whether they had a preoperative seizure or not and serum levels were achieved days before surgery.
Anesthetic and surgical technique
*Awake group
Awake craniotomy under local anesthesia and monitored conscious sedation protocol was used. The patient was comfortably positioned with a warm-air blanket to avoid shivering. Continuous intraoperative monitoring was done using electrocardiogram, pulse oximeter, and noninvasive arterial blood pressure monitors. Bi-spectral index (BIS) was also used to monitor the level of consciousness. Oxygen (2 L/min) was administered through a nasal cannula. Intravenous propofol (1.5 - 2.5 mg/kg) and fentanyl (1 μg/kg) were administered to tolerate the circumferential scalp block to the supratrochlear, supraorbital, zygomaticotemporal, auriculotemporal, greater occipital, and lesser occipital nerves. The local anesthetic was bupivacaine-lidocaine mixture with adjuvants as Mg sulfate and dexamethasone as previously reported by our team.[
*GA group
For patients who underwent surgery under GA, same monitors were used as in awake patients. The primary anesthetic concern for those patients is the avoidance of halogenated inhaled agents, which can increase the latency and decrease the amplitude of evoked potentials. In addition, chemical muscle relaxants must be avoided. Propofol-based TIVA was used. Initial bolus dose of propofol (1.52.5 mg/kg) was given plus fentanyl (1 μg/kg) then endotracheal tube or laryngeal mask airway of an appropriate size was applied. Maintenance doses of propofol (6–12 mg/kg/h) with fentanyl (25 μg) bolus every 30 min regularly. BIS index was maintained between 40 and 60, and during mapping was around 60. Motor evoked potentials (motor evoked potential), phase reversals were attached to the patients before surgery, stimulation was started with 4 mA increased to 20 mA maximum with strip electrode for ECoG as in awake cases.
*Both groups
Standard microsurgical resection techniques were performed either for awake or GA cases. Intraoperative ultrasonography (EUB-405 plus ultrasound scanner, HITACHI) was used in all cases for locating the lesions, choosing the shortest route, defining their margins, and evaluating the EOR. Once cortical mapping had been done, the area of cortical resection was then outlined. The main aim in all cases was maximal resection with minimal neurological deficit. The tumor boundary close to the eloquent areas was kept to be resected last. If functional impairment occurred, either clinical for awake cases or decreased MEPs for GA cases, the resection would be stopped. If the impairment was confirmed and did not improve within 5 min after exclusion of other factors of impairment, the resection would not be resumed. If the impairment subsided, continuation or termination of the resection was dependent on senior surgeon decision according to nature of tumor and prior discussion with the patient.
Management of intraoperative stimulation-induced seizure: (For all cases)
Ice-cold saline or Ringer’s lactate was always available for cortical irrigation in case of any seizure. Mapping was stopped for 5 min and sedatives were avoided for further successful mapping. If prolonged or recurrent seizure was encountered, small doses of propofol or/and midazolam were given with reload of antiepileptic medication. Emergent airway strategies as laryngeal mask airway or endotracheal tube were in place (for awake cases) in case of sudden complications.
Postoperative course and follow-up
All patients were sent to ICU for monitoring and transferred to the general ward once stable. Postcraniotomy standard treatments were prescribed including steroids and dehydrating measures for cerebral edema, antiepileptic medications, and analgesia. Same antiepileptic medications were kept postoperative as pre-operatively for at least 1 month, and then their antiepileptics and dosages were managed in conjunction with neurologist according to preoperative seizures history and intra/postoperative seizures. Postoperative evaluation of neurological outcome was done at 3 different times: (1) after the surgery, when the patients could be fully evaluated and (2) at the 1- and 3-month follow-up visits. Early postoperative MRI with contrast was performed in all cases within 72 h and at 3 months follow-up to document the EOR using (3D Slicer version 4 software, BWH and 3D Slicer contributors) by independent neuroradiologist blinded to clinical and operative data. EOR was graded as following: gross total resection (GTR) indicated more than 98% resection; near total resection when there was more than 90% resection, subtotal resection when there was 50–90% resection, and partial resection (PR) when it was less than 50%.
Statistical analysis
All data were collected, tabulated, and statistically analyzed using SPSS v.23.0.0 (IBM Corp., Armonk, New York, United States). Quantitative data were expressed as mean or median (range), and qualitative data were expressed as absolute frequencies (number) and relative frequencies (percentage). Percent of categorical variables was compared using a Pearson Chi-square test when appropriate. Risk assessment was done by relative risk and confidence interval 95% (CI 95%). A logistic regression univariate model was used to assess for significant predictors of intraoperative stimulation-induced seizures then significant variables were assessed in the multivariate logistic regression model. P < 0.05 was considered statistically significant.
RESULTS
Patients and tumor characteristics
Sixty-four patients with perirolandic glioma underwent surgical resection using intraoperative cortical and subcortical mapping techniques between 2014 and 2019. Of these patients, 40 patients (62.5%) were operated with awake craniotomy, while 24 patients (37.5%) underwent surgery under GA. The mean age for awake group was 42.7 ± 14.5 years and 49.5 ± 15.5 years for GA group (P = 0.08). There were (65%) males in awake group and (41.7%) in GA group (P = 0.69). All patients had a KPS of 70 or more before surgery. The most presenting symptoms were headaches (75% awake group, 58.3% GA group; P = 0.16), seizures (47.5% awake group, 45.8% GA group; P = 0.92), and motor weakness (45% awake group, 58.3% GA group; P = 0.3) [
Twenty-four patients of awake group (60%) had right sided lesion versus 14 patients (58.3%) in GA group. The mean preoperative tumor volume for the awake group was 36.6 ± 17.3 cm3 and for the GA group was 45.3 ± 20.9 cm3 (P = 0.07). Glioblastoma multiforme GIV was the most encountered pathology in both groups and presented (37.5%) in awake group and (45.8%) in GA group (P = 0.51), [
The mean EOR in awake cases was 92.03% and 90.05% in GA cases (P = 0.23). GTR> 98% was achieved in 18 patients (45%) in awake group and 7 patients (29.2%) in GA group (P = 0.2). None of cases in both groups had PR below 50% [
Intraoperative stimulation-induced seizures
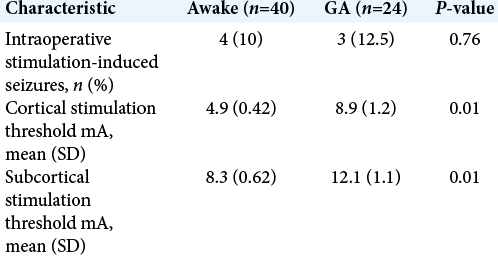
In the awake group, the mean cortical stimulation intensity needed to evoke motor responses was significantly lower than the intensity in GA patients (4.9 ± 0.42 vs. 8.9 ± 1.2 mA, respectively, P = 0.01). Furthermore, subcortical stimulation threshold was statistically significant lower in awake patients (8.3 ± 0.62 vs. 12.1 ± 1.1 mA, respectively, P = 0.01) [
Intraoperative cortical stimulation-induced seizures in 7 patients in both groups (10.9%), 4 patients in awake group (10%) experienced focal seizures, and 2 of them were controlled with only ice-cold ringer’s lactate cortical irrigation while the other 2 patients needed small doses of sedation, but none of those patients had been converted to be operated under GA and further mapping was completed successfully [
Figure 1:
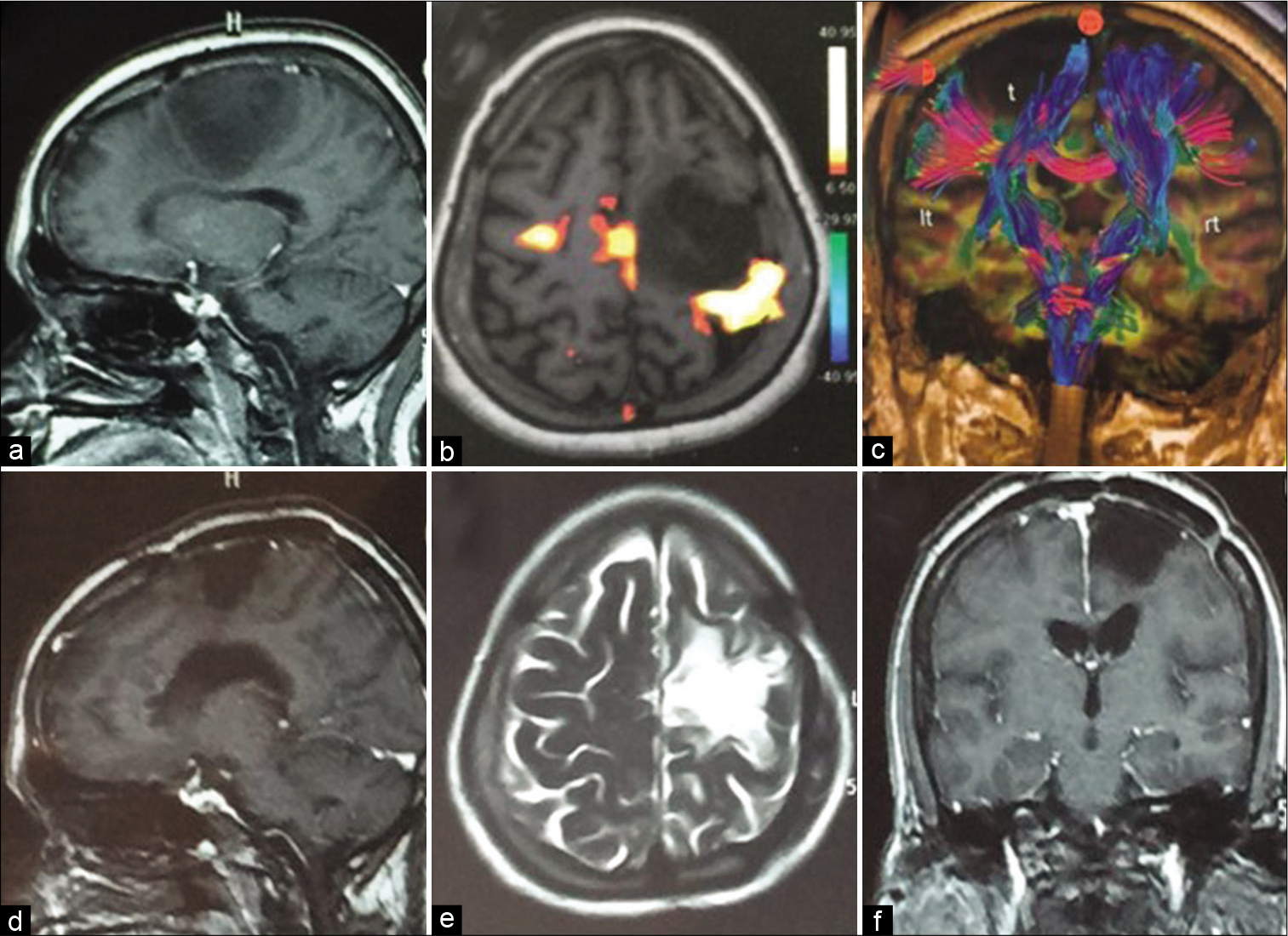
A case of left perirolandic fibrillary astrocytoma grade II operated by awake craniotomy and mapping techniques with occurrence of intraoperative stimulation-induced focal seizures controlled by ice-cold ringer’s lactate irrigation and further mapping was successfully completed with gross total resection and postoperative uneventful recovery without new deficit, (a) preoperative MRI brain (sagittal T1 with contrast), (b and c) preoperative fMRI and DTI, (d-f) 3 months postoperative follow-up MRI brain with contrast.
Postoperative characteristics
Eleven patients in the GA group (45.8%) encountered immediate postoperative worsened or new motor deficit, compared to 15 patients (37.5%) in the awake group (P = 0.51). At 3 months follow-up, only 2 patients (5%) of awake group had permanent deficit versus 2 patients (8.3%) who had operation under GA (P = 0.59). Postoperative seizures occurred in 9 awake patients (22.5%) and 8 GA patients (33.3%) (P = 0.34). Two patients in the GA group and 3 awake patients had small tumor bed hematomas which were treated conservatively, while only 1 patient in the GA group who developed postoperative large hematoma which required evacuation at the same operative day with subsequent good recovery. One patient from GA group had deep venous thrombosis and one had postoperative urinary tract infection. Three patients (4.7%) (2 awake and one from GA group) had postoperative wound infection which required conservative treatment with no further surgical interventions.
Predictors for intraoperative stimulation-induced seizures
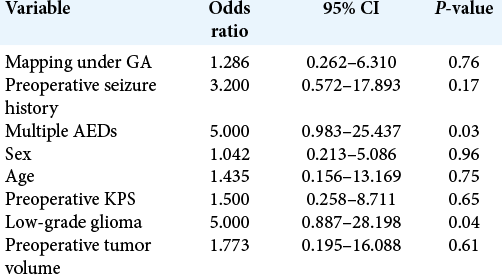
Factors that might predict intraoperative seizures, while using cortical and subcortical stimulation mapping techniques were evaluated through logistic regression analysis. The univariate analysis revealed that patients who were treated preoperatively with multiple antiepileptic drugs (AEDs) (P = 0.03) and those with low-grade glioma (P =0.04) were statistically significant predictors but did not show significance on multivariate analysis. Type of operation either awake or under GA, age, sex, preoperative KPS, preoperative seizure history, and preoperative tumor volume were not significant predictors [
DISCUSSION
A large amount of retrospective data in the literature proved the value of maximum resection of glial-type tumors and suggested that the median survival time and time to recurrence are improved in patients who undergo aggressive resection either for low or high grade types;[
Intraoperative motor mapping and monitoring can be performed while patient is awake or under GA, and still it is unclear which approach is preferable as little previous studies compared between both approaches.[
In the current study, immediate postoperative worsened or new motor deficit was reported higher in GA group (45.8% vs. 37.5% in awake patients) but was not statistically significant (P = 0.51), Zelitzki et al.[
Furthermore, Brallier et al.[
Intraoperative stimulation-induced seizures may result in difficulties in further mapping and affect surgical outcome and EOR, and in awake craniotomy, either mild inconvenience to the patient and surgeon up to uncontrolled generalized seizures necessitating conversion to GA can happen. Intraoperative seizures occurred in 10% of our awake patients and 12.5% of GA cases; although it is statistically insignificant, the higher incidence in GA cases can be due to the higher cortical stimulation threshold needed to evoke motor response, we need further studies and higher patients number to confirm this point. Eseonu et al.[
Study limitations
The limitations of this study are those inherent to retrospective nature with small populations. One of the inclusion criteria for awake craniotomy is the patient’s acceptance to perform such technique which is source for a selection bias. This study does not account for the experience gained by the team and evolved by years for better deal with such cases.
CONCLUSION
Intraoperative cortical and subcortical mapping techniques increase safety and efficacy of perirolandic glioma resection either patient is awake or under GA with low incidence of stimulation-induced seizures which do not affect the procedure or further mapping. Low-grade glioma and previous use of multiple AEDs can be considered as predictors for higher incidence of stimulation-induced seizures. Awake craniotomy can be done safely in selected patients with continuous clinical assessment throughout the resection, lower stimulation intensities required for mapping and lower but statistically nonsignificant incidence of intraoperative seizures.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Boetto J, Bertram L, Moulinié G, Herbet G, Moritz-Gasser S, Duffau H. Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: Electrocorticography is not mandatory. World Neurosurg. 2015. 84: 1838-44
2. Brallier JW, Dalal PJ, McCormick PJ, Lin HM, Deiner SG. Elevated intraoperative serum lactate during craniotomy is associated with new neurological deficit and longer length of stay. J Neurosurg Anesthesiol. 2017. 29: 388-92
3. Chacko AG, Thomas SG, Babu KS, Daniel RT, Chacko G, Prabhu K. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013. 115: 329-34
4. Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M. When gross total resection of a glioblastoma is possible, how much resection should be achieved?. World Neurosurg. 2014. 82: e257-65
5. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014. 16: 113-22
6. Charras P, Herbet G, Deverdun J, de Champfleur NM, Duffau H, Bartolomeo P. Functional reorganization of the attentional networks in low-grade Glioma patients: A longitudinal study. Cortex. 2015. 63: 27-41
7. Chen MW, Morsy AA, Liang S, Ng WH. Re-do craniotomy for recurrent grade IV glioblastomas: Impact and outcomes from the national neuroscience institute Singapore. World Neurosurg. 2016. 87: 439-45
8. Cirillo S, Caulo M, Pieri V, Falini A, Castellano A. Role of functional imaging techniques to assess motor and language cortical plasticity in Glioma patients: A systematic review. Neural Plast. 2019. 2019: 4056436
9. Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, van Effenterre R. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005. 76: 845-51
10. Duffau H. The anatomo-functional connectivity of language revisited: New insights provided by electrostimulation and tractography. Neuropsychologia. 2008. 46: 927-34
11. Duffau H. The dangers of magnetic resonance imaging diffusion tensor tractography in brain surgery. World Neurosurg. 2014. 81: 56-8
12. Eseonu CI, Rincon-Torroella J, Lee YM, ReFaey K, Tripathi P, Quinones-Hinojosa A. Intraoperative seizures in awake craniotomy for perirolandic Glioma resections that undergo cortical mapping. J Neurol Surg A Cent Eur Neurosurg. 2018. 79: 239-46
13. Eseonu CI, Rincon-Torroella J, ReFaey K, Lee YM, Nangiana J, Vivas-Buitrago T. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: Evaluating perioperative complications and extent of resection. Neurosurgery. 2017. 81: 481-9
14. Fakhri M, O’Donnell LJ, Rigolo L, Golby AJ.editors. Mapping eloquent brain with functional MRI and DTI. Functional Mapping of the Cerebral Cortex. Cham: Springer; 2016. p. 41-62
15. Gonen T, Grossman R, Sitt R, Nossek E, Yanaki R, Cagnano E. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J Neurosurg. 2014. 121: 1133-8
16. Grossman R, Ram Z. Awake craniotomy in Glioma surgery. Eur Assoc Neuro Oncol Mag. 2013. 4: 27-33
17. Hamer PD, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on Glioma surgery outcome: A meta-analysis. J Clin Oncol. 2012. 30: 2559-65
18. Han SJ, Morshed RA, Troncon I, Jordan KM, Henry RG, Hervey-Jumper SL. Subcortical stimulation mapping of descending motor pathways for perirolandic gliomas: Assessment of morbidity and functional outcome in 702 cases. J Neurosurg. 2018. 131: 201-8
19. Hervey-Jumper SL, Berger MS. Role of surgical resection in low-and high-grade gliomas. Curr Treat Options Neurol. 2014. 16: 284
20. Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012. 308: 1881-8
21. Kavouridis VK, Boaro A, Dorr J, Cho EY, Iorgulescu JB, Reardon DA. Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: A volumetric analysis. J Neurosurg. 2019. 133: 1291-301
22. Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006. 105: 34-40
23. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013. 54: 12-7
24. Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009. 64: 836-46
25. Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the state-trait anxiety inventory and the Zung self-rating depression scale. Br J Clin Psychol. 1983. 22: 245-9
26. McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008. 63: 700-8
27. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009. 65: 463-70
28. Nasr YM, Waly SH, Morsy AA. Scalp block for awake craniotomy: Lidocaine-bupivacaine versus lidocainebupivacaine with adjuvants. Egypt J Anaesth. 2020. 36: 7-15
29. Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T. Intraoperative seizures during awake craniotomy: Incidence and consequences: Analysis of 477 patients. Neurosurgery. 2013. 73: 135-40
30. Ohtaki S, Akiyama Y, Kanno A, Noshiro S, Hayase T, Yamakage M. The influence of depth of anesthesia on motor evoked potential response during awake craniotomy. J Neurosurg. 2017. 126: 260-5
31. Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014. 120: 846-53
32. Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: Increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015. 38: E3
33. Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011. 68: 1192-8
34. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008. 62: 753-66
35. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults: A review. J Neurosurg. 2011. 1: 1-8
36. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
37. Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol. 2000. 21: 1055-63
38. Serletis D, Bernstein M. Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg. 2007. 107: 1-6
39. Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008. 26: 1338-45
40. Tan LA, Byrne RW, Sturaitis MK.editors. Anesthetic considerations in cortical mapping and awake surgery. Functional Mapping of the Cerebral Cortex. Cham: Springer; 2016. p. 77-90
41. Zelitzki R, Korn A, Arial E, Ben-Harosh C, Ram Z, Grossman R. Comparison of motor outcome in patients undergoing awake vs general anesthesia surgery for brain tumors located within or adjacent to the motor pathways. Neurosurgery. 2019. 85: E470-6