- Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, Utah, USA
- Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah, USA
- Department of Pathology, Division of Anatomic Pathology, University of Utah, Salt Lake City, Utah, USA
- Department of Neuroradiology, University of Utah, Salt Lake City, Utah, USA
- Department of Radiology, Texas Tech University Health Sciences Center, El Paso, Texas, USA
Correspondence Address:
Randy L. Jensen
Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, Utah, USA
Huntsman Cancer Institute, University of Utah, Salt Lake City, Utah, USA
DOI:10.4103/sni.sni_116_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Nicholas T. Gamboa, Michael Karsy, Joseph T. Gamboa, Nam K. Yoon, Meghan J. Driscoll, Joshua A. Sonnen, Karen L. Salzman, Randy L. Jensen. Preoperative and intraoperative perfusion magnetic resonance imaging in a RELA fusion-positive anaplastic ependymoma: A case report. 24-Jul-2018;9:144
How to cite this URL: Nicholas T. Gamboa, Michael Karsy, Joseph T. Gamboa, Nam K. Yoon, Meghan J. Driscoll, Joshua A. Sonnen, Karen L. Salzman, Randy L. Jensen. Preoperative and intraoperative perfusion magnetic resonance imaging in a RELA fusion-positive anaplastic ependymoma: A case report. 24-Jul-2018;9:144. Available from: http://surgicalneurologyint.com/surgicalint-articles/preoperative-and-intraoperative-perfusion-magnetic-resonance-imaging-in-a-rela-fusion%e2%80%91positive-anaplastic-ependymoma-a-case-report/
Abstract
Background:Ependymomas are rare neuroepithelial tumors thought to arise from radial glial precursor cells lining the walls of the ventricles and central canal of the brain and spinal cord, respectively. Histopathological classification, according to World Health Organization criteria, has only recently defined the RELA-fusion positive ependymoma. These tumors may account for 70% of supratentorial ependymomas in children and represent an aggressive entity distinct from other ependymomas.
Case Description:Here we present the case of a patient with RELA-fusion positive ependymoma of the frontal lobe in whom we used preoperative and intraoperative magnetic resonance (MR) perfusion imaging. In this first demonstrated intraoperative evaluation of MR perfusion in ependymoma, increased peripheral perfusion of the lesion in a ring-like manner with a discrete cutoff around the surgical margin correlated with intraoperative findings of a clear border between the tumor and brain, as well as pathological findings of increased MIB index and hypercellularity—specifically within solid tumor components. An abnormal perfusion pattern also suggested an aggressive lesion, which was later confirmed on pathological analysis. In addition, intraoperative MR perfusion improved detection of tumor tissue in combination with traditional T1-weighted contrast-enhanced methods, which increased extent of resection.
Conclusions:MR perfusion imaging may be a useful method for delineating tumor aggressiveness and borders, which can be prognostic.
Keywords: Brain tumor, ependymoma, imaging, neurosurgery, perfusion magnetic resonance imaging, RELA fusion-positive
INTRODUCTION
Ependymoma is the third most common primary brain tumor in children.[
The 2016 World Health Organization (WHO) classification formally introduced RELA-positive ependymomas, which result from chromothripsis of chromosome 11 resulting in a C11orf95–RELA fusion protein.[
Imaging of RELA-positive ependymomas often demonstrates a large mass that appears heterogeneous, with both solid and cystic components, but definitive diagnosis with conventional imaging alone remains challenging.[
CASE REPORT
History and examination
A previously healthy 19-year-old man presented with a 6-week history of word-finding difficulty and a 3-week history of headaches, nausea, and vomiting, but he was otherwise neurologically intact. Diagnostic T1-weighted imaging with contrast revealed a heterogeneously enhancing lesion in the left frontal lobe with significant mass effect and surrounding vasogenic edema, initially concerning for an astrocytoma or oligodendroglioma [
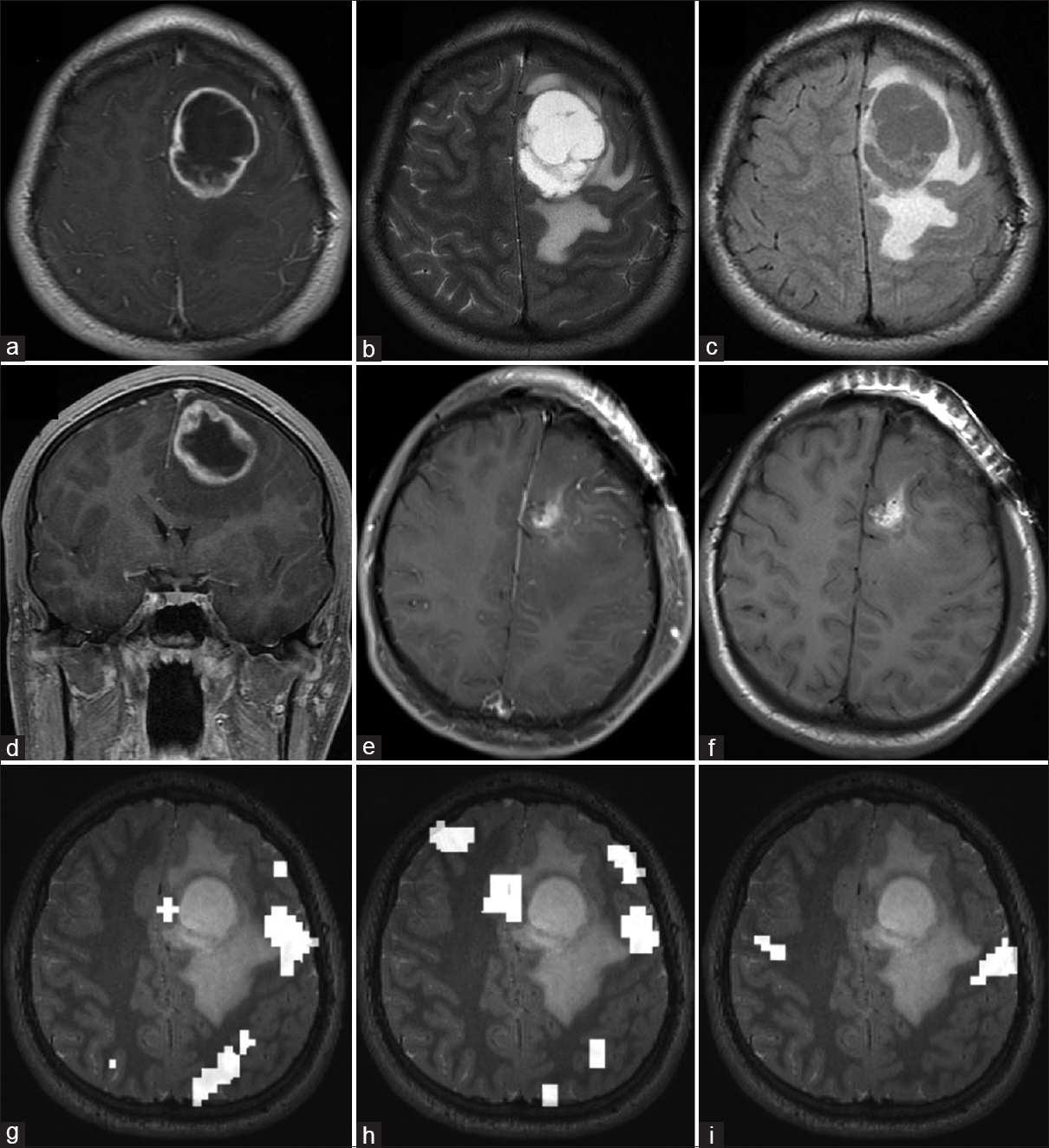
Figure 1
Preoperative and postoperative MRI of RELA-fusion positive ependymoma. Preoperative axial (a) T1-weighted gadolinium-enhanced, (b) T2-weighted, (c) FLAIR, and (d) coronal T1-weighted contrast-enhanced imaging demonstrate a ring-enhancing lesion with cystic central component and perilesional edema in the left frontal cortex. Postoperative (e) T1-weighted contrast-enhanced and (f) T1-weighted nonenhanced imaging show a gross total resection. Functional MRI data for (g) reading, (h) word recognition, and (i) lip movement are shown. Compared with the tumor, language cortex localizes posterolateral while motor cortex is further posterior
MR perfusion was performed in addition to standard preoperative and intraoperative imaging sequences, including T1-, T2-, and diffusion-weighted imaging (DWI), fluid-attenuated inverse resonance imaging (FLAIR), gradient and spin echo imaging (GSE), and contrast-enhanced imaging as described earlier.[
Operative and postoperative course
The patient underwent a stereotactic left frontal craniotomy and tumor resection with neuromonitoring and iMRI. Conventional intraoperative studies (T1 with and without contrast, T2, FLAIR, DWI/ADC, stereotactic T1) demonstrated gross total resection [
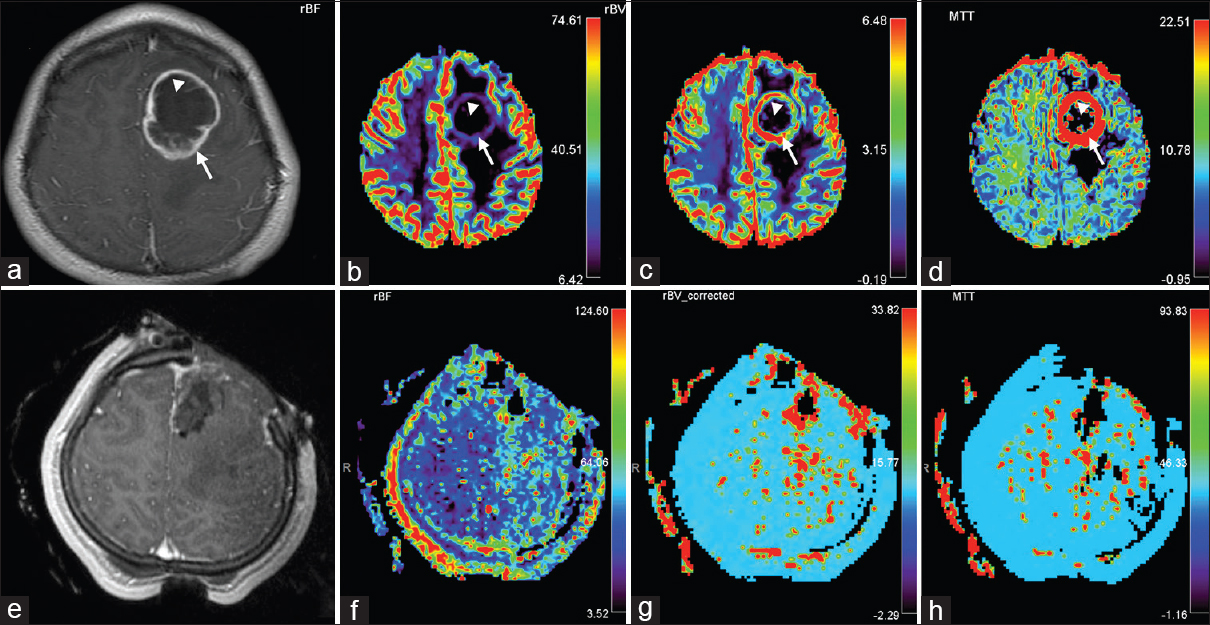
Figure 2
Preoperative and intraoperative MR perfusion of RELA-fusion positive ependymoma. Preoperative (a) T1-weighted with contrast, (b) CBF, (c) CBV, and (d) mean transit time (MTT) imaging demonstrates increased diffusion in the ring-enhancing portion of the tumor, suggestive of an aggressive lesion (arrow). This perilesional area (arrow) correlated with the solid tumor component seen on pathological analysis [
Pathological analysis
The final pathological results of the original tumor and biopsy of lateral, posterior, and deep resection cavity margins were consistent with RELA-fusion positive anaplastic ependymoma [
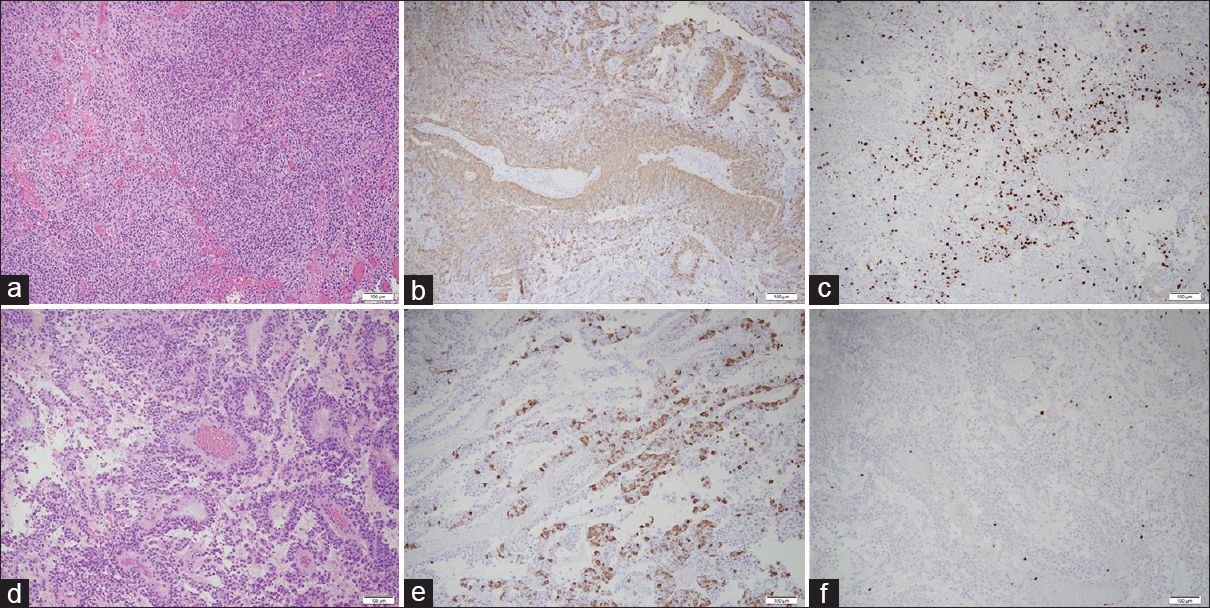
Figure 3
Pathological analysis of RELA-fusion positive ependymoma. (a, d) The hematoxylin and eosin-stained tissue showed two morphologic patterns to the tumor. Some areas of the tumor consist of solid sheets of tumor cells (a–c), whereas other areas of the tumor have papillary architecture (d and f). The tumor nuclei are round and regular with evenly distributed chromatin. Areas with increased cellularity, predominately in the solid areas of the tumor, show up to six mitotic figures in ten high-power fields. Small foci of tumor necrosis (not pictured) are also identified. A prominent vascular network is present in the tumor with fibrotic vessels. The tumor cells cuff the vessels, although there is a lack of an acellular zone containing fibrillary projections. (b and e) The immunohistochemical stain for GFAP shows patchy positivity in the tumor cells, with the tumor cells surrounding the vessels being notably positive. (c and f) The MIB-1/Ki-67 stain is positive in approximately 60% of the tumor nuclei in the solid areas of the tumor (c), and occasional nuclei are positive in the papillary areas (f). The MIB-1/Ki-67 index overall is greater than 10% in the tumor. The immunohistochemical stain for IDH-1 is negative (not pictured). All photomicrographs taken at 100× total magnification
DISCUSSION
The 2016 WHO classification of ependymomas not only focuses primarily on histopathology but also incorporates a new genetically defined subtype—RELA-fusion positive ependymoma.[
Ependymomas are often heterogeneous masses with cystic components, necrotic change, calcification, and hemorrhage. In particular, supratentorial intraparenchymal ependymomas can be highly variable in appearance and can be difficult to distinguish from other glial neoplasms with conventional imaging modalities such as computed tomography (CT) and MRI.[
Imaging
Most intracranial ependymomas (60%) are located in the infratentorial posterior fossa and classically arise from the floor of the fourth ventricle and may exude through the foramina of Luschka and Magendie.[
MR perfusion has been shown to be helpful in the identification of more aggressive lesions. Delgado et al.[
Limitations
Because this represents only a single case report with no other cases available for comparison, it is possible that the MR perfusion signal was artifactual. Further study of preoperative and intraoperative MR perfusion as a modality to understand glial tumor heterogeneity is currently underway at our institution.
CONCLUSION
RELA-positive ependymoma is a newly defined clinical entity. Preoperative and perfusion iMRI could be used to better understand the progression of this tumor. Within the ring-enhancing rim of the tumor, increased tumor perfusion and vascularity was observed. In addition, this perfusion area correlated with the more aggressive, solid tumor components and a corresponding higher MIB index than other tumor parts. Perfusion iMRI also aided the resection of additional tumor. Additional studies with perfusion iMRI may aid in the identification of characteristic imaging findings that may be of diagnostic and prognostic significance in the preoperative setting.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barajas RF, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009. 253: 486-96
2. Cachia D, Wani K, Penas-Prado M, Olar A, McCutcheon IE, Benjamin RS. C11orf95-RELA fusion present in a primary supratentorial ependymoma and recurrent sarcoma. Brain Tumor Pathol. 2015. 32: 105-11
3. Delgado AF, Delgado AF. Discrimination between glioma grades II and III using dynamic susceptibility perfusion MRI: A meta-analysis. AJNR Am J Neuroradiol. 2017. 38: 1348-55
4. Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011. 10: 7-
5. Figarella-Branger D, Lechapt-Zalcman E, Tabouret E, Junger S, de Paula AM, Bouvier C. Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro Oncol. 2016. 18: 919-27
6. Grill J, Pascal C, Chantal K. Childhood ependymoma: A systematic review of treatment options and strategies. Paediatr Drugs. 2003. 5: 533-43
7. Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014. 16: 280-91
8. Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML. Gliomas: Predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008. 247: 490-8
9. Lee CH, Chung CK, Ohn JH, Kim CH. The similarities and differences between intracranial and spinal ependymomas: A review from a genetic research perspective. J Korean Neurosurg Soc. 2016. 59: 83-90
10. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Mack SC, Pajtler KW, Chavez L, Okonechnikov K, Bertrand KC, Wang X. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature. 2018. 553: 101-5
12. Murayama K, Nishiyama Y, Hirose Y, Abe M, Ohyu S, Ninomiya A. Differentiating between central nervous system lymphoma and high-grade glioma using dynamic susceptibility contrast and dynamic contrast-enhanced mr imaging with histogram analysis. Magn Reson Med Sci. 2018. 17: 42-9
13. Onishi S, Yamasaki F, Nakano Y, Takayasu T, Amatya VJ, Kolakshyapati M. RELA fusion-positive anaplastic ependymoma: Molecular characterization and advanced MR imaging. Brain Tumor Pathol. 2018. 35: 41-5
14. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C. CBTRUS Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015. 17: iv1-iv62
15. Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015. 27: 728-43
16. Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014. 506: 451-5
17. Petrella JR, Provenzale JM. MR perfusion imaging of the brain: Techniques and applications. AJR Am J Roentgenol. 2000. 175: 207-19
18. Pietsch T, Wohlers I, Goschzik T, Dreschmann V, Denkhaus D, Dorner E. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-kappaB signaling pathway. Acta Neuropathol. 2014. 127: 609-11
19. Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I. From the radiologic pathology archives: intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics. 2013. 33: 21-43
20. Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005. 8: 323-35
21. Vera-Bolanos E, Aldape K, Yuan Y, Wu J, Wani K, Necesito-Reyes MJ. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015. 17: 440-7
22. Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. 2009. 25: 1203-13