- Department of Neurosurgery, Instituto de Oncología Ángel H Roffo,
- Department of Diagnosis, Pathology, Instituto de Oncología Ángel H Roffo,
- Department of Neuro-Oncology, Oncology, Instituto de Oncología Ángel H Roffo,
- Department of Neurosurgery, “José María Ramos Mejía” General Hospital, Buenos Aires, Argentina.
Correspondence Address:
Dr. Martin Andres Merenzon, Department of Neurosurgery, Instituto de Oncología Ángel H Roffo, Buenos Aires, Argentina.
DOI:10.25259/SNI_373_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Martin Andres Merenzon1, Jose Ignacio Gómez Escalante2, Diego Prost3, Eduardo Seoane4, Alejandro Mazzon1, Érica Rojas Bilbao2. Preoperative imaging features: Are they useful tools for predicting IDH1 mutation status in gliomas Grades II–IV?. 05-Aug-2022;13:332
How to cite this URL: Martin Andres Merenzon1, Jose Ignacio Gómez Escalante2, Diego Prost3, Eduardo Seoane4, Alejandro Mazzon1, Érica Rojas Bilbao2. Preoperative imaging features: Are they useful tools for predicting IDH1 mutation status in gliomas Grades II–IV?. 05-Aug-2022;13:332. Available from: https://surgicalneurologyint.com/surgicalint-articles/11774/
Abstract
Background: It is already known that gliomas biomolecular parameters have a reliable prognostic value. However, an invasive procedure is required to determine them. Our aim was to better understand the clinical characteristics of gliomas Grades II–IV and to assess the usefulness of imaging features in magnetic resonance imaging (MRI) to predict the isocitrate dehydrogenase one (IDH1) mutation.
Methods: Preoperative MRI characteristics were retrospectively reviewed and molecular diagnosis of gliomas was tested in adult patients between 2014 and 2021 in two institutions. We applied a biological criterion to divide the brain in cerebral compartments.
Results: A total of 108 patients met the inclusion criteria. Contrast enhancement (CE) in MRI was significantly associated with wild-type IDH1 (IDH1-Wt) (P
Conclusion: Our research revealed that CE is useful for predicting IDH1-Wt in gliomas. On the contrary, nonCE is not useful for predicting IDH1-Mut gliomas. Thus, the traditional concept of associating non-CE MRI with a low-grade glioma should be reviewed, as it can lead to an underestimation of the potential aggressiveness of the tumor. If this association was validated with the future prospective studies, a noninvasive tool would be available for predicting gliomas IDH1 mutation status.
Keywords: Glioma, Isocitrate dehydrogenase, Limbic system, Molecular diagnosis, Paralimbic system, Radiological features
INTRODUCTION
The 2016 and 2021 World Health Organization (WHO) central nervous system (CNS) tumor classification generated a conception shift on how to diagnose and understand gliomas by adding molecular diagnosis, such as isocitrate dehydrogenase (IDH) mutation or 1p19q codeletion status, to the classic histologic classification. It is already known that these biomolecular parameters have a reliable prognostic value.[
There is evidence that gliomas have a predilection for the frontal, temporal, and insular lobe.[
We hypothesize that imaging characteristics correlate with biological behavior of gliomas which is, in turn, strongly related to their genotype. In this study, we investigate whether there is an association between IDH1 mutation status and several radiological characteristics. The location of the lesions was defined in a novel way according to the division in cerebral functional compartments proposed by Yasargil,[
MATERIALS AND METHODS
The study has been approved by both Institutional Research Ethics Committees before experiment was started and has been conducted in accordance with the principles set forth in the Helsinki Declaration of 1975, as revised in 2000.
Patients’ selection and study design
An observational, epidemiological, retrospective, and multicenter study was designed.
Patients with a diagnosis of cerebral glioma between January 2014 and December 2021 were identified and analyzed. All the data were obtained from the records of both institutions, which are publicly funded. All patients included were adults, underwent biopsy or tumor resection, and had an MRI (1.5 or 3T) available for review done before their first surgery. Patients’ age was considered at the date of their first surgery. Accessible neuroimaging consisted of at least T2-weighted, FLAIR, T1-weighted, and T1-weighted postcontrast sequences. Patients with ependymomas and choroid plexus tumors were excluded from the study.
The cases diagnosed between 2014 and 2020 according to the 2007 or 2016 WHO classification were reviewed at the Institute of Oncology “Ángel H. Roffo” by a single neuropathologist (JIGE). Molecular testing was done and the diagnosis was reclassified according to the 2021 WHO classification.
Molecular analysis
All genetic testing was done at the Department of Diagnosis of the Institute “Roffo.” IDH1 mutation analysis was performed by immunohistochemistry using a monoclonal antibody against IDH1-R132H (H09, Dianova, Hamburg, Germany) in all specimens. Furthermore, by immunohistochemistry, alpha-thalassemia/mental retardation X-linked (ATRX) expression (AX1 clon, Dianova, Hamburg, Germany), p53 staining (D07 clon, Leica, Buffalo Grove, United States of America), and glial fibrillary acidic protein (EP672Y clon, Roche-Ventana, Oro Valley, United States of America) were tested. Finally, CDKN2A homozygous deletion and 1p/19q codeletion analysis were performed by fluorescence in situ hybridization by means of double detection with a specific DNA molecular probe (ZytoLight SPEC CDKN2A/ CEN 9 and 1p36/1q25 and 19q13/19q13 dual color probe, Bremerhaven, Germany). The latter was tested in all gliomas except in glioblastomas (GBMs). This decision was based on the diagnostic algorithm proposed by the WHO.[
Imaging characteristics
Preoperative contrast-enhanced MRIs were reviewed. Patients’ images were classified as “CE” or “non-CE” according to the T1-weighted postcontrast sequences. “CE” was defined as any contrast-enhancing nonvascular lesion, when compared with the noncontrast T1 images, regardless of its pattern of enhancement or appearance. In addition, tumor borders were categorized as “sharp” or “indistinct.”
Two of the authors evaluated the images independently, and when their decisions were not concordant, they met to discuss and agreed on the final classification.
Topography
Due to the infiltrative nature of gliomas, when there was a single lesion that did not enhance with contrast, the tumor topography was considered where the epicenter of the lesion in the T2-weighted and FLAIR sequences was located. In gliomas that did enhance with gadolinium, their location was considered according to the epicenter of the tumor in the T1-weighted postcontrast sequences.[
The tumors’ cerebral topography was classified as follows, according to the functional compartments proposed by Yasargil et al.:[
If one lesion occupied two or more compartments, approximately equal in volume, without normal brain interposed, the topography was named “combined.”
A glioma was classified as “multicentric” if the patient presented two or more lesions in any given compartment with normal brain interposed between them.
An “eloquent area” was defined as a cortical cerebral area whose function cannot be replaced in case of injury (e.g., the primary sensorimotor area, speech centers, or insula).
Finally, the side of the lesion was systemized as “left,” “right,” or “bilateral.”
Statistical analysis
Data were analyzed through a descriptive analysis. The association between imaging features and IDH1 mutation status was tested using the Chi-squared test or Fisher’s exact test on occasions when frequencies were <5. In all tests, a two-tailed P < 0.05 was considered statically significant.
RESULTS
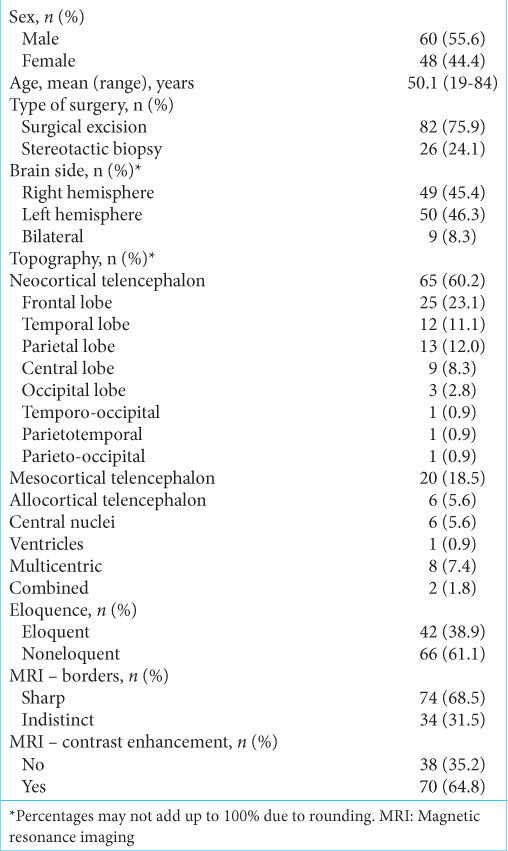
Of 111 patients initially evaluated, 108 met the inclusion criteria, 55.6% were male, with a mean age of 50.1 years (19–84 years). About 75.9% of the samples were obtained by resection, 24.1% by biopsy. The most frequent diagnosis was GBM IDH1-Wt (58/108 = 53.7%). Only considering gliomas Grades II–III, diffuse astrocytoma, IDH1-Mut/ATRX-Mut (11/108 = 10.2%) was the most frequent. There were 13 cases categorized as diffuse gliomas NOS, all of them Grade II and IDH1-Wt. Five of them were midline gliomas, located in the central nuclei compartment. The other Grade II (n =20) gliomas were IDH1-Mut. Finally, 9/15 (60%) of the Grade III gliomas were IDH1-Mut. The patients’ characteristics and neuroimaging findings are summarized in
Regarding tumor topography, 60.2% were localized in the neocortical telencephalon (23.1% in the frontal lobe and 11.1% in the temporal lobe), 24.1% in the paralimbic and limbic system, and 7.4% were multicentric. Only 4.6% of all gliomas involved two or more different compartments. In our investigation, no cerebellar gliomas were reported.
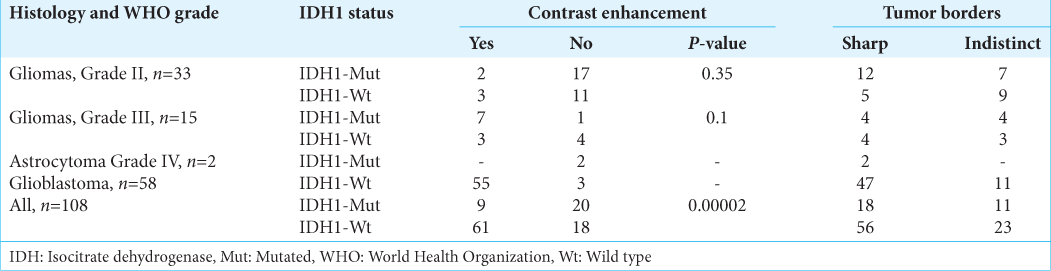
Addressing the hypothesis of our research, we looked for an association between the molecular profile and imaging characteristics in presurgical MRI. In
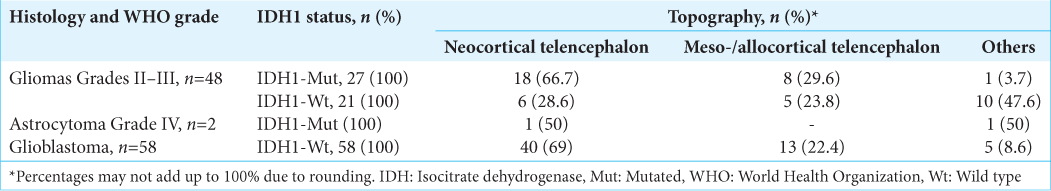
When analyzing a possible association between genotype and topography, considering Grades II–III gliomas [
DISCUSSION
In this investigation, we found that CE is statistically associated with an IDH1-Wt, in low- and high-grade gliomas. Given its positive predictive value of 87.1%, it can be considered a useful characteristic for predicting an IDH1-Wt. On the contrary, according to our results, non-CE is not a good predictor of an IDH1-Mut. This is because the negative predictive value, that is, the probability that the patient has a mutated IDH1 tumor since the MRI does not show CE, was of 52.6%. This is relevant given the general impression of inferring a low-grade glioma from lesions that do not enhance with gadolinium in the MRI. Taking this evidence into account is that this concept should be reviewed and that gliomas that do not enhance with contrast should not be underestimated. In summary, no CE is not a good feature for predicting IDH1-Mut gliomas.
It is already known that the presence of CE in gliomas is an imaging characteristic of aggressive tumors[
We observed that most of the gliomas (65/108 = 60.2%) have their epicenter in the neocortical telencephalic compartment, keeping this distribution even considering Grades II–III and GBM separately. It is known that gliomas have a predilection for the frontal, temporal, and insular lobe.[
We highlight an investigation that compares the molecular profile of gliomas located in the temporal lobe neocortex with others located in the meso- and allocortex of the mentioned lobule.[
This finding, without having statistical power, is also observed in our research when we found that IDH1-Mut Grades II–III glial tumors have a higher prevalence in the neocortical compartment than in other compartments and that IDH1-Wt, a lower prevalence. One possible explanation proposes that since the glia of different brain regions expresses different growth factors, and oncogenesis occurs in cells that overexpress a specific growth factor, then tumors would only grow in a particular region.[
This study has several limitations. The first is that only the IDH1-R132H mutation was evaluated due to the lack of availability in publicly funded institutions in Argentina of the specific techniques for evaluation of other IDH1 and IDH2 mutations. Fortunately, the R132H mutation in the IDH 1 gene represents 95%[
Despite the potential limitations, we consider that the greatest value of this work is focused on the positive finding of CE as a predictor of IDH1-Wt. Furthermore, we highlight the questioning of the concept of inferring a good prognosis glioma due to the fact that the lesion does not enhance with contrast in the MRI. Therefore, continuing in the future, the investigation of a possible association between gliomas genotype and radiological features would allow us to provide a noninvasive clinical prognosis instrument that could also have an impact on therapeutic strategies.
CONCLUSION
CE was a reliable imaging feature for predicting IDH1-Wt gliomas. On the other hand, non-CE did not allow predicting with certainty the IDH1 status. According to our study, the concept that if a glioma does not enhance with contrast which is of good prognosis should be reviewed. Furthermore, most gliomas were located in the neocortical telencephalon. In addition, with the exception of very aggressive tumors, gliomas grew within the limits of a single brain compartment.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
This work was supported by the National Cancer Institute, Ministry of Health, Argentina [grant numbers 14/2018] and the Florencio Fiorini Foundation [grant 2020], Buenos Aires, Argentina and publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Antonio Colobraro, M.D. for collaboration on histologic diagnosis, María Florencia Soto for providing language help and Jimena Vicens, M.D. for her contribution to the statistical analysis.
References
1. Castet F, Alanya E, Vidal N, Izquierdo C, Mesia C, Ducray F. Contrast-enhancement in supratentorial low-grade gliomas: A classic prognostic factor in the molecular age. J Neurooncol. 2019. 143: 515-23
2. Darlix A, Deverdun J, Menjot de Champfleur N, Castan F, Zouaoui S, Rigau V. IDH mutation and 1p19q codeletion distinguish two radiological patterns of diffuse low-grade gliomas. J Neurooncol. 2017. 133: 37-45
3. Darlix A, Gozé C, Rigau V, Bauchet L, Taillandier L, Duffau H. The etiopathogenesis of diffuse low-grade gliomas. Crit Rev Oncol Hematol. 2017. 109: 51-62
4. Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004. 100: 2622-6
5. Gozé C, Mansour L, Rigau V, Duffau H. Distinct IDH1/IDH2 mutation profiles in purely insular versus paralimbic WHO Grade II gliomas. J Neurosurg. 2013. 118: 866-72
6. Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996. 27: 65-73
7. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
8. Larjavaara S, Mäntylä R, Salminen T, Haapasalo H, Raitanen J, Jääskeläinen J. Incidence of gliomas by anatomic location. Neuro Oncol. 2007. 9: 319-25
9. Lasocki A, Tsui A, Gaillard F, Tacey M, Drummond K, Stuckey S. Reliability of noncontrast-enhancing tumor as a biomarker of IDH1 mutation status in glioblastoma. J Clin Neurosci. 2017. 39: 170-5
10. Li H, Ren X, Zhang J, Lin S. Mediobasal and lateral temporal gliomas exhibit different growth patterns, surgical outcomes and prognoses. Clin Neurol Neurosurg. 2015. 133: 90-5
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
12. Merenzon MA, Escalante JI, Prost D, Seoane E, Mazzón A, Bilbao ÉR. Algorithm for the integrated diagnosis of gliomas 2021. Our experience. Medicina (B Aires). 2022. 82: 370-5
13. Metellus P, Coulibaly B, Colin C, de Paula AM, Vasiljevic A, Taieb D. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010. 120: 719-29
14. Michiwaki Y, Hata N, Mizoguchi M, Hiwatashi A, Kuga D, Hatae R. Relevance of calcification and contrast enhancement pattern for molecular diagnosis and survival prediction of gliomas based on the 2016 World Health Organization Classification. Clin Neurol Neurosurg. 2019. 187: 105556
15. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013. 19: 764-72
16. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2013-2017. Neuro Oncol. 2020. 22: iv1-96
17. Pierallini A, Bonamini M, Bozzao A, Pantano P, Stefano DD, Ferone E. Supratentorial diffuse astrocytic tumours: Proposal of an MRI classification. Eur Radiol. 1997. 7: 395-9
18. Prost DM, Merenzon MA, Gómez-Escalante JI, Primavera A, Benítez MV, Gil AS. Effects of time to chemoradiation on high-grade gliomas from the Buenos Aires Metropolitan Area. PLoS One. 2021. 16: e0249486
19. Ren X, Cui X, Lin S, Wang J, Jiang Z, Sui D. Co-deletion of chromosome 1p/19q and IDH1/2 mutation in glioma subsets of brain tumors in Chinese patients. PLoS One. 2012. 7: e32764
20. Sonoda Y, Shibahara I, Kawaguchi T, Saito R, Kanamori M, Watanabe M. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol. 2015. 32: 99-104
21. Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: A systemic review and meta-analysis. Eur Radiol. 2019. 29: 745-58
22. 23. Yamauchi T, Ohno M, Matsushita Y, Takahashi M, Miyakita Y, Kitagawa Y. Radiological characteristics based on isocitrate dehydrogenase mutations and 1p/19q codeletion in grade II and III gliomas. Brain Tumor Pathol. 2018. 35: 148-58 24. Yaşargil MG, von Ammon K, Cavazos E, Doczi T, Reeves JD, Roth P. Tumours of the limbic and paralimbic systems. Acta Neurochir (Wien). 1992. 118: 40-52 25. Yaşargil MG. Microneurosurgery of CNS Tumor. Stuttgart, New York: New York: Georg Thieme Verlag, Thieme Medical Publishers; 1994. Vol 4: 26. Zaninovich RS, Jalon PG, Golan JN. Gliomas: Aspectos biológicos, clínicos y terapéuticos [Gliomas: Biologic, clinic and therapeutic features]. Buenos Aires. 2017. 1: 17-45 27. Zlatescu MC, TehraniYazdi A, Sasaki H, Megyesi JF, Betensky RA, Louis DN. Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res. 2001. 61: 6713-5