- Gamma Knife of Spokane, 910 W 5th Ave, Suite 102, Spokane, WA 99204, USA
- Cancer Care Northwest, 910 W 5th Ave, Suite 102, Spokane, WA 99204, USA
- University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195, USA
- Inland Neurosurgery and Spine Associates, 105 W 8th Ave, Suite 200, Spokane, WA 99204, USA
- Department of Neurosurgery, Rockwood Clinic, 801 W 5th Ave, Suite 525, Spokane, WA 99204, USA
- DataWorks Analytics, LLC, 3952 N Magnuson St, Coeur D’Alene, ID 83815, USA
Correspondence Address:
Christopher M. Lee
Gamma Knife of Spokane, 910 W 5th Ave, Suite 102, Spokane, WA 99204, USA
Cancer Care Northwest, 910 W 5th Ave, Suite 102, Spokane, WA 99204, USA
DOI:10.4103/2152-7806.194065
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrew T. Roehrig, Ethan A. Ferrel, Devon A. Benincosa, Alexander R. MacKay, Benjamin C. Ling, Jonathan D. Carlson, John J. Demakas, Aaron Wagner, Wayne T. Lamoreaux, Robert K. Fairbanks, Jason A. Call, Barton S. Cooke, Ben Peressini, Christopher M. Lee. Pretreatment clinical prognostic factors for brain metastases from breast cancer treated with Gamma Knife radiosurgery. 14-Nov-2016;7:
How to cite this URL: Andrew T. Roehrig, Ethan A. Ferrel, Devon A. Benincosa, Alexander R. MacKay, Benjamin C. Ling, Jonathan D. Carlson, John J. Demakas, Aaron Wagner, Wayne T. Lamoreaux, Robert K. Fairbanks, Jason A. Call, Barton S. Cooke, Ben Peressini, Christopher M. Lee. Pretreatment clinical prognostic factors for brain metastases from breast cancer treated with Gamma Knife radiosurgery. 14-Nov-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/pretreatment-clinical-prognostic-factors-brain-metastases-breast-cancer-treated-gamma-knife-radiosurgery/
Abstract
Background:Brain metastases significantly affect morbidity and mortality rates for patients with metastatic breast cancer. Treatment for brain metastases lengthens survival, and options such as stereotactic radiosurgery (SRS) can increase survival to 12 months or longer. This study retrospectively analyzes the prognostic factors for overall survival (OS) for patients with one or multiple brain metastases from breast cancer treated with SRS.
Methods:Between December 2001 and May 2015, 111 patients with brain metastases from breast cancer were grouped by potential prognostic factors including age at diagnosis, Karnofsky Performance Status (KPS) score, number of brain metastases, and whether or not they received adjuvant treatments such as whole brain radiotherapy (WBRT) or surgical resection. Survival rates were determined for all groups, and hazard ratios were calculated using univariate and multivariate analyses to compare differences in OS.
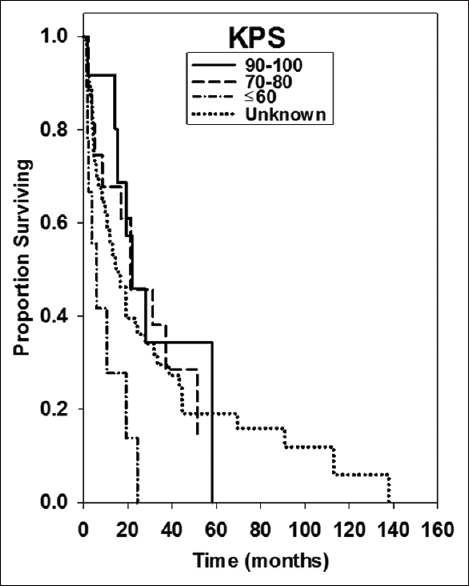
Results:Median OS was 16.8 ± 4.22 months. Univariate analysis of patients with a KPS ≤60 and multivariate analysis of KPS 70–80 showed significantly shorter survival than those with KPS 90–100 (5.9 ± 1.22 months, 21.3 ± 11.69 months, and 22.00 ± 12.56 months, P = 0.024 and P = 0.779).
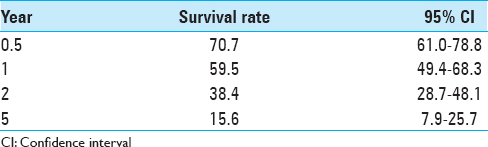
Conclusion:SRS has been shown to be safe and effective in treating brain metastases from breast cancer. We found our median survival to be 16.8 ± 4.22 months, an increase from other clinical reports. In addition, 38.4% of our population was alive at 2 years and 15.6% survived 5 years. Significant prognostic factors can help inform clinical treatment decisions. This study found that KPS was a significant prognostic indicator of OS in these patients.
Keywords: Brain metastases, breast cancer, stereotactic radiosurgery
INTRODUCTION
Brain metastases are the number one cause of morbidity and mortality for approximately 1.4 million Americans who are diagnosed with cancer every year. An estimated 20%–40% of those diagnosed with cancer will develop brain metastases over the course of their illness.[
The clinical approach to managing brain metastases comprises one or several modalities including chemotherapy, surgical resection, WBRT, and stereotactic radiosurgery (SRS). The effectiveness of radiosurgery in treating brain metastases has been well documented, and several methods are available for delivery of radiation.[
To date, there are several retrospective studies that outline some prognostic factors associated with radiosurgery treatment of BCBM.[
In this study, we offer 111 retrospectively analyzed data from patients with BCBM treated with Gamma Knife radiosurgery (GKRS) at Gamma Knife of Spokane. Our goal is to outline several pretreatment clinical factors that have effects on prognosis for patients undergoing treatment.
MATERIALS AND METHODS
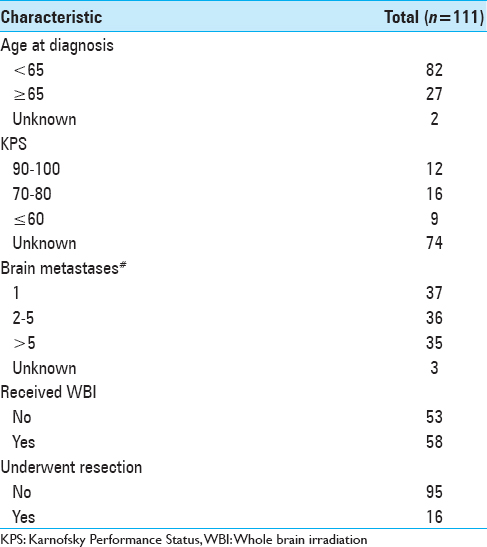
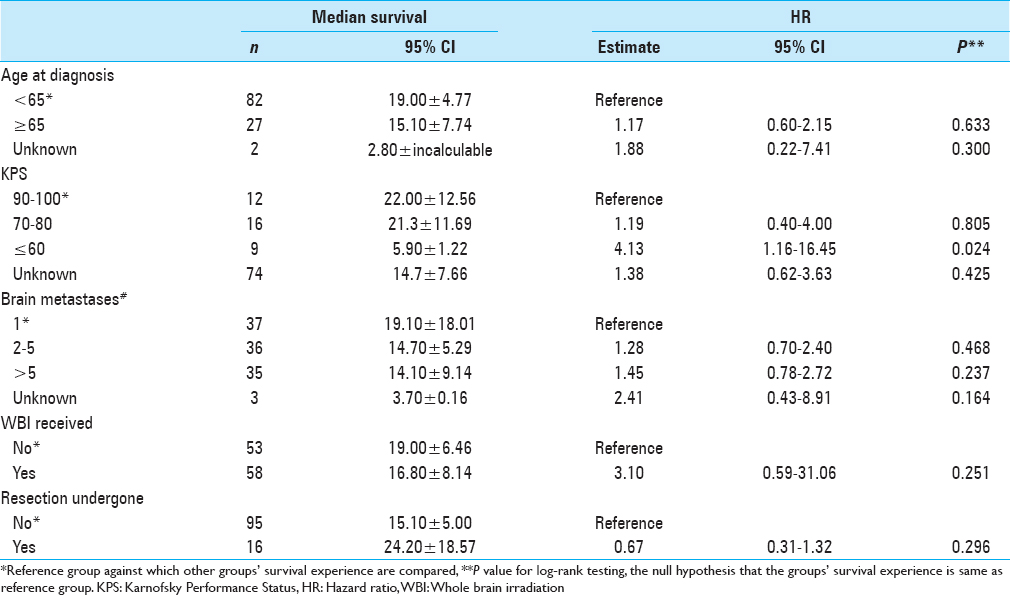
For this analysis, we examined 111 patients with a diagnosis of brain metastasis and primary tumor histology of breast cancer. These patients were treated between December 2001 and May 2015 by physicians at Gamma Knife of Spokane. The patients were grouped by age at brain metastasis diagnosis (<65, 65+) by whether or not they received whole brain irradiation (WBI), by whether or not they underwent resection, by KPS value, and by the number of brain metastases (1, 2–5, >5). Number of patients in these groups are shown in
Survival curves were estimated using the Kaplan-Meier method and used to compare treatment groups, age groups, KPS groups, and brain metastases number groups. Andersen confidence intervals of 95% were constructed for the median survival time of the groups. An estimate of the standard error was used to calculate approximate confidence intervals for the log-hazard ratio (HR).
To determine whether there is statistical evidence of differences between the survival curves of the groups, log-rank tests were employed. The multivariate analysis of the treatment groups, age groups, KPS groups, and brain metastases number groups used the Cox proportional hazard model. All statistical analyses utilized StatsDirect version 2.8.0 (StatsDirect Ltd., Altrincham, UK) and SigmaPlot version 11.0 (SYSTAT Software, Inc., San Jose, CA, USA).
RESULTS
A total of 111 patients were included in the study. Their baseline characteristics are shown in
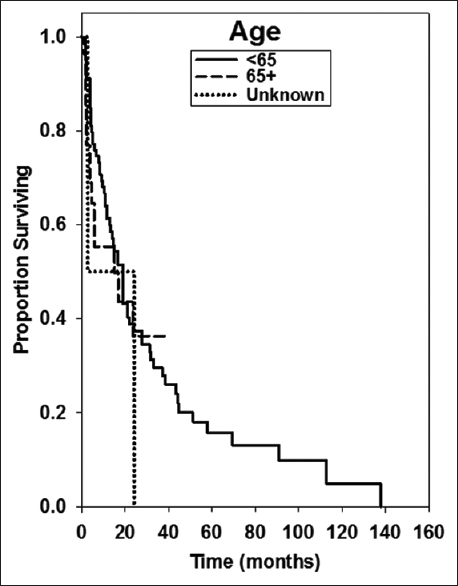
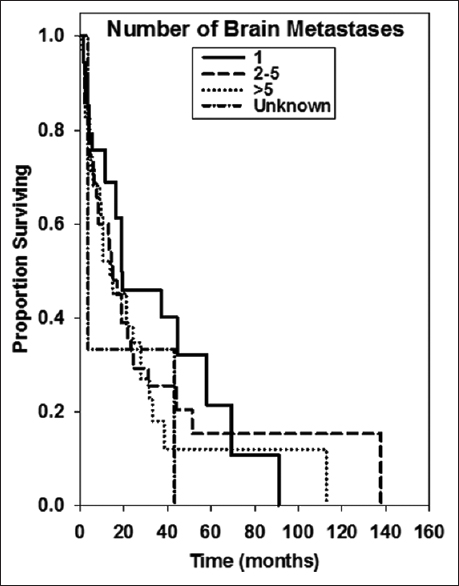
Median OS was 16.8 ± 4.22 months. Kaplan–Meier survival curves for several treatment groups are found in Figures
Univariate median survival confidence interval and HR confidence intervals are shown in
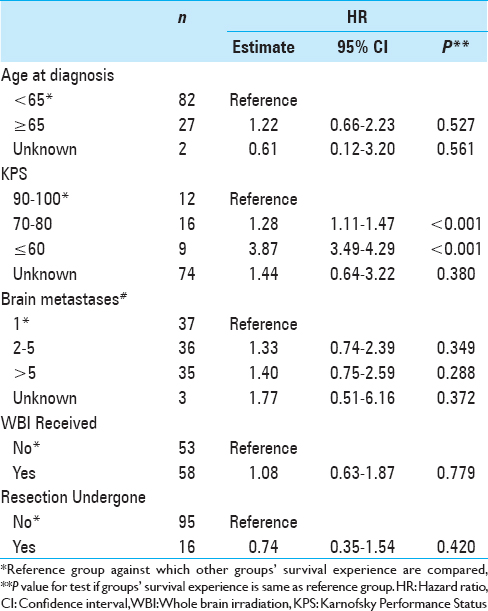
The multivariate analysis HR estimates and confidence intervals are shown in
Fifty-eight (52%) patients received WBRT in addition to SRS treatment. Median survival lengths for patients who did and did not receive WBRT were 16.8 ± 8.14 months and 19.0 ± 6.46 months, respectively. This was not a significant difference upon multivariate or univariate analysis (P = 0.779 and 0.251, respectively).
Absolute survival rates are shown in
DISCUSSION
Brain metastases are less common than bone or other soft tissue metastases for patients with breast cancer, but they represent a significantly poorer prognosis and are often resistant to systemic therapies.[
Our study found that KPS score was a significant prognostic factor. Patients with lower KPS score on the day of treatment had significantly shorter survival than did those with high functionality. This is consistent with other reports of KPS as a strong predictor of survival for patients undergoing SRS. Kased et al. found that KPS score was a significant prognostic factor in both univariate and multivariate analyses for the 176 patients treated with radiosurgery or radiosurgery plus WBRT.[
Age >60 or 65 has been found by some studies to be predictive of shorter OS following the diagnosis of brain metastases, although it generally seems to be a less reliable predictor than KPS score or RPA class indicators.[
Breast cancer-specific prognostic factors may differ from those of other tumor types, according to recent studies differentiating BCBM OS from other intracranial metastases. In 2012, Sperduto et al. published seminal work describing a modified graded prognostic assessment (GPA) for breast-specific cancers.[
Our multivariate survival analyses also looked at adjuvant therapies over the course of the patient's treatment, specifically the addition of surgical resection or WBRT to SRS. The literature is generally in agreement that while the addition of SRS to WBRT may improve intracranial recurrence rates, there is no significant difference in OS for patients with brain metastases from varying tumor etiologies.[
Our study found that there was no significant difference in OS between patients who received WBRT or surgical resection treatments in addition to SRS (P = 0.779 and P = 0.42, respectively). This finding may have biases typically associated with retrospective studies, such as that patients who received WBRT may have had more brain metastases or patients who received surgical resection may have had fewer metastases that were surgically accessible. This finding is also in agreement with the breast-GPA model developed by Sperduto et al. Although the GPA guidelines are intended to inform treatment decisions, they analyzed the data with intact treatment modalities and found no significant difference in median survival time.[
Radiosurgery is also recently being used for patients with a greater number of metastases, and deferral of WBRT has become the standard of care for many patients.[
Presence of extracranial disease is often used as a prognostic indicator for brain metastasis OS. In breast cancer, this prognostic value may be limited in part due to the success of systemic therapy. This is especially true since the era of trastuzumab therapy for HER2-positive breast cancers. For intracranial metastases, low levels of trastuzumab penetrate the blood–brain barrier, but this effect can be somewhat overcome after treatment with WBRT.[
Our study is inherently limited due to its retrospective nature. The patients who were selected for GKRS may have had better prognosis than other BCBM patients who were ineligible for SRS treatment. Because this study is not a randomized controlled trial, it has limited scope for informing clinical practice. It does, however, suggest that patients with BCBM who have good performance status (KPS ≥70) may have increased survival, despite other negatively influencing factors such as age ≥65 or multiple brain metastases. In addition, KPS scores were only available for 37 patients (33%), even though this remained our strongest predictive factor.
CONCLUSION
Our study suggests that patients with BCBM who have good performance status (KPS ≥70) may have increased survival, despite other negatively influencing factors such as age ≥65 or multiple brain metastases. In this report, the best OS rates were statistically associated with a KPS of 90 or greater. We did not find any significant survival benefit to the addition of WBRT or surgery to SRS treatment on univariate or multivariate analysis. We found encouraging long-term survival for some patients, with 15% of our cohort reaching the 5-year survival mark.
Each BCBM patient and his/her family should be informed of all treatment options and of the risks and benefits associated with each. Individual preferences and goals should also be a considered factor for physicians treating BCBM patients. SRS therapy provides the most benefit for patients with good performance status upon diagnosis of brain metastases. Repeated treatments may also be beneficial for patients who continue to have good performance status and a low gross tumor volume. GKRS continues to be a safe and effective treatment option to manage brain metastases with focal radiotherapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Eric Reynolds and Jill Adams for their help and support, as well as the entire Gamma Knife of Spokane and Cancer Care Northwest research staff, for their contributions to this manuscript.
References
1. Akyurek S, Chang EL, Mahajan A, Hassenbusch SJ, Allen PK, Mathews LA. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007. 30: 310-4
2. Amendola BE, Wolf AL, Coy SR, Amendola M, Bloch L. Gamma knife radiosurgery in the treatment of patients with single and multiple brain metastases from carcinoma of the breast. Cancer J. 2000. 6: 88-92
3. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006. 295: 2483-91
4. Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007. 68: 1388-95
5. Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014. 23: 623-8
6. Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013. 14: 64-71
7. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009. 10: 1037-44
8. Dawood S, Gonzalez-Angulo AM, Albarracin C, Yu TK, Hortobagyi GN, Buchholz TA. Prognostic factors of survival in the trastuzumab era among women with breast cancer and brain metastases who receive whole brain radiotherapy: A single-institution review. Cancer. 2010. 116: 3084-92
9. deAzevedo Santos TR, Tundisi CF, Ramos H, Maia MA, Pellizzon AC, Silva ML. Local control after radiosurgery for brain metastases: Predictive factors and implications for clinical decision. Radiat Oncol. 2015. 10: 63-
10. Diener-West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys. 1989. 16: 669-73
11. Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: The importance of HER-2 status. Cancer. 2008. 112: 2359-67
12. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37: 745-51
13. Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005. 75: 5-14
14. Goyal S, Prasad D, Harrell F, Matsumoto J, Rich T, Steiner L. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg. 2005. 103: 218-23
15. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: An alternative to whole brain radiotherapy?. Neurosurgery. 2003. 52: 1318-26
16. Hayashi N, Niikura N, Masuda N, Takashima S, Nakamura R, Watanabe K. Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: A multicenter retrospective analysis. Breast Cancer Res Treat. 2015. 149: 277-84
17. Iuchi T, Shingyoji M, Sakaida T, Hatano K, Nagano O, Itakura M. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013. 82: 282-7
18. Kased N, Binder DK, McDermott MW, Nakamura JL, Huang K, Berger MS. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys. 2009. 75: 1132-40
19. Klos KJ, O’Neill BP. Brain metastases. Neurologist. 2004. 10: 31-46
20. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011. 29: 134-41
21. Kondziolka D, Kano H, Harrison GL, Yang HC, Liew DN, Niranjan A. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. J Neurosurg. 2011. 114: 792-800
22. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004. 22: 3608-17
23. Muacevic A, Kreth FW, Tonn JC, Wowra B. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. 2004. 100: 1705-11
24. Newton HB. Chemotherapy for the treatment of metastatic brain tumors. Expert Rev Anticancer Ther. 2002. 2: 495-506
25. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat Oncol. 2014. 9: 155-
26. Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: Differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010. 21: 942-8
27. Rades D, Kueter JD, Veninga T, Gliemroth J, Schild SE. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT SRS) versus surgery plus whole brain radiotherapy (OP WBRT) for 1-3 brain metastases: Results of a matched pair analysis. Eur J Cancer. 2009. 45: 400-4
28. Serna A, Escolar PP, Puchades V, Mata F, Ramos D, Gömez MA. Single fraction volumetric modulated arc radiosurgery of brain metastases. Clin Transl Oncol. 2015. 17: 596-603
29. Shultz DB, Modlin LA, Jayachandran P, Von Eyben R, Gibbs IC, Choi CY. Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for new brain metastases after initial SRS. Int J Radiat Oncol Biol Phys. 2015. 92: 993-9
30. Shuto T, Fujino H, Inomori S, Nagano H. Repeated gamma knife radiosurgery for multiple metastatic brain tumours. Acta Neurochir (Wien). 2004. 146: 989-93
31. Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002. 53: 519-26
32. Soffietti R, Rudà R, Trevisan E. Brain metastases: Current management and new developments. Curr Opin Oncol. 2008. 20: 676-84
33. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012. 82: 2111-7
34. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007. 18: 23-8
35. Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005. 167: 913-20
36. Yamamoto M, Kawabe T, Sato Y, Higuchi Y, Nariai T, Watanabe S. Stereotactic radiosurgery for patients with multiple brain metastases: A case-matched study comparing treatment results for patients with 2-9 versus 10 or more tumors. J Neurosurg. 2014. 121: 16-25