- Department of Radiology, Naresuan University, Tha Pho, Thailand.
- Department of Surgery Buddhashinnaraj Hospital, Mueang, Phitsanulok, Thailand.
- Department of Radiology, Buddhashinnaraj Hospital, Mueang, Phitsanulok, Thailand.
- Department of Surgery, Naresuan University, Tha Pho, Thailand.
Correspondence Address:
Peeraphong Thiarawat, Department of Surgery, Naresuan University, Tha Pho, Phitsanulok, Thailand.
DOI:10.25259/SNI_741_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Waneerat Galassi1, Warin Yuyangkate2, Paweena Paholthep1, Thipsumon Tangsriwong3, Ponnarong Jaikon1, Thongchai Leiwan4, Jiroje Jiranukool4, Peeraphong Thiarawat4. Prevalence of unruptured intracranial aneurysms among first-degree relatives of Thai patients who had aneurysmal subarachnoid hemorrhage. 23-Nov-2021;12:566
How to cite this URL: Waneerat Galassi1, Warin Yuyangkate2, Paweena Paholthep1, Thipsumon Tangsriwong3, Ponnarong Jaikon1, Thongchai Leiwan4, Jiroje Jiranukool4, Peeraphong Thiarawat4. Prevalence of unruptured intracranial aneurysms among first-degree relatives of Thai patients who had aneurysmal subarachnoid hemorrhage. 23-Nov-2021;12:566. Available from: https://surgicalneurologyint.com/surgicalint-articles/11249/
Abstract
Background: The prevalence of familial unruptured intracranial aneurysm (UIA) in Thai population was unknown.
Methods: Our study population comprised first-degree relatives of patients who were diagnosed with aneurysmal subarachnoid hemorrhage (aSAH) in two cerebrovascular neurosurgical centers from January 2018 to December 2018. The volunteers underwent three-dimensional time-of-flight magnetic resonance angiography for screening intracranial aneurysms (IA). Those who were reported positive or suspected of IA then underwent computed tomography angiography for confirmation.
Results: We identified 12 patients who had 12 unruptured IAs (UIAs) from among 93 first-degree relatives. The prevalence of UIA among our study population was 12.9%. An estimated prevalence of UIA among Thai population was 9.05% (95% confidence interval [CI] 7.32–10.78). Of the 93 relatives, 84 had only one first-degree relative who suffered aSAH. Siblings posed a higher risk for UIA than offspring (16% vs. 9.5%), but the difference was not statistically significant (odds ratio 1.810, 95% CI 0.50–6.50, P = 0.274). The most common aneurysm location was the anterior cerebral artery territory (50%).
Conclusion: The prevalence of familial UIA in a Thai population was relatively high. There was no significant between-group difference in the occurrence of UIA between the siblings and offspring of the aSAH patients.
Keywords: First-degree relative, Prevalence, Screening magnetic resonance angiography, Unruptured intracranial aneurysm
INTRODUCTION
The prevalence of intracranial aneurysms (IAs) has been reported to range from 1% to 3% according to autopsy and population-based studies in normal populations.[
The prevalence of familial aneurysm has been shown to vary among different races; Japanese,[
MATERIALS AND METHODS
We conducted a prospective study in two institutions located in the northern part of Thailand. These two cerebrovascular centers are the sole aneurysm centers for 4 million catchment residents. The study populations comprised all of the first-degree relatives of patients with aSAH from January 2018 to December 2018. Eligible volunteers who agreed to participate in this project were included consecutively. Exclusion criteria included patients with: a previous diagnosis of IA, at least one previous brain MRA or computed tomography angiography (CTA), first-degree relatives who had an unknown cause of stroke, and contraindications for magnetic resonance imaging, such as, pregnancy, metallic instrument implantation, pacemakers, and claustrophobia.
Demographic data, risk factors, genetic diseases, and compressive aneurysm symptoms were collected by interview and a review of the medical records. However, our routine institutional practice does not investigate genetic diseases; thus, genetic disease data may be underreported. Three-dimensional time-of-flight MR angiography was performed using two MR machines. The first hospital used a 1.5 T MR scanner (Ingenia 1.5T, Philips Medical Systems) using the following parameters: TR 23 msec, TE 6.9 msec, and FOV 200 × 200 × 176 mm3. The second hospital used a 1.5 T MR scanner (Magnetom Symphony 1.5 T, Siemen Medical Systems) using the following parameters: TR 24 msec, TE 6.0 msec, and FOV 190 × 166.3 × 117 mm3.
The MRA data of all patients were reviewed by two radiologists and confirmed independently by a neuroradiologist. Confirmatory CTA was performed for patients suspected of a IA. This study was approved by Naresuan University Institutional Review Board (NU-IRB No. 180/57). Informed consent was acquired from all of the volunteers. We confirmed that all methods were carried out in accordance with the approval protocols and regulations.
We used IBM SPSS for Macintosh (IBM Inc., Armonk, New York) version 23.0.0 for the statistical analysis. For the quantitative variables, the mean, median, and percentages were measured. Fisher’s exact test was used to identify between-group differences among the factors related to the presence of UIAs. P < 0.05 was considered statistically significant. We calculated an estimated prevalence using calculating method for imperfect test (due to high false positives of CTA) described by Greenland to decrease biases.[
RESULTS
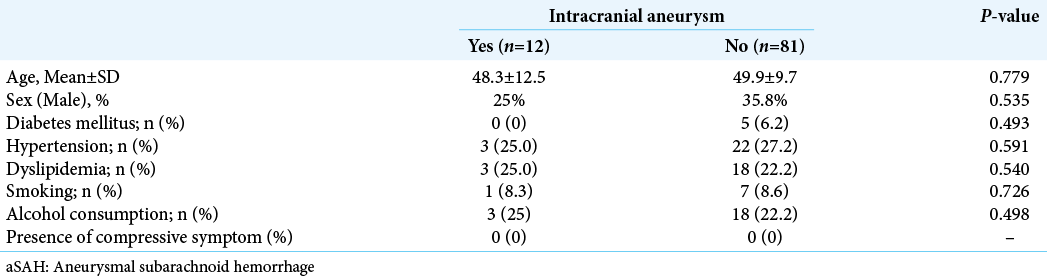
During the study period, 96 patients suffered from aSAH. Ninety-three first-degree relatives of the patients who were included in this study were identified. Of the 93 relatives, 32 underwent MRA screening at the first hospital, and 61 underwent MRA screening at the second hospital. Patient demographic data are shown in [
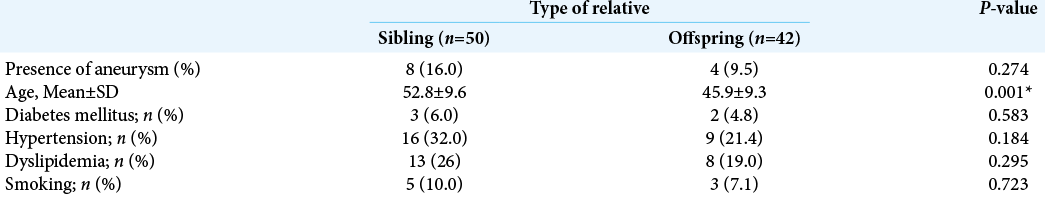
Three-dimensional time-of-flight MRA detected a suspected aneurysm in 18 of the relatives. Confirmatory CTA revealed that 12 had UIA, whereas six did not. Thus, the prevalence of UIA among first-degree relatives in this population was 12.9%. The estimated prevalence of familial UIA among Thai populations was 9.05% (95% confidence interval [CI] 7.32– 10.78). Siblings appeared to have a higher prevalence of IA than offspring (16.0% vs. 9.5%), but the difference was statistically insignificant (odds ratio [OR] 1.810, 95% CI 0.50–6.50, P = 0.274), as shown in [
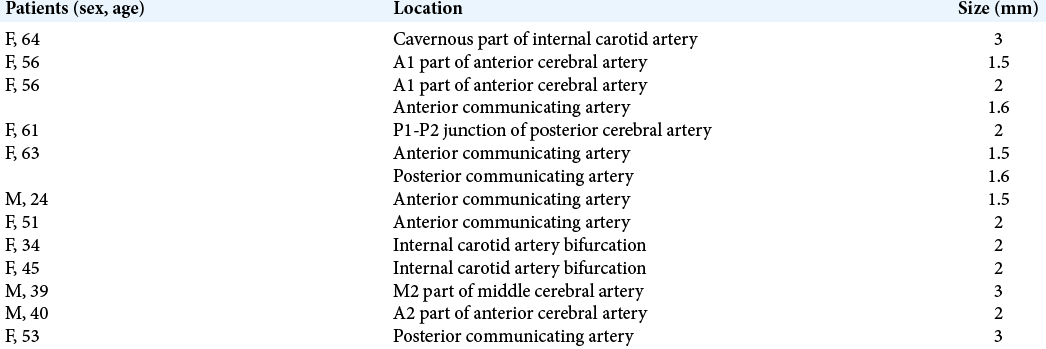
Fourteen UIAs were identified among the 12 patients. Of these, 13 were located in the anterior circulations. The most common aneurysm location was the anterior cerebral artery territory [
DISCUSSION
Screening MRA for UIA is recommended when two or more first-degree relatives have aneurysms or aSAH.[
A previous study revealed an increasing trend for UIA with an increasing number of affected first-degree relatives.[
Thein et al. reported that the most common aneurysm location in Singaporeans was the cavernous part of the internal carotid arteries, whereas that of our patients was the anterior cerebral artery territories.[
From our study, the positive predictive value (PPV) of three-dimensional time-of-flight MR angiography was 66.6%. Raaymaker et al. revealed a PPV of 58% when a possible aneurysm was interpreted from MRA, which is comparable to our study.[
The major limitation of this study was the small sample size. Thus, the statistical power might not be adequate to detect the true prevalence of IA. In addition, only patients who were suspected of having aneurysms proceeded to undergo confirmatory CTA. Thus, the sensitivity and negative predictive value could not be evaluated. Finally, we did not perform digital subtraction angiography. Our mean aneurysm size was 2 ± 0.5 mm that might be within the limits of CTA. Thus, the effective prevalence could not be reported.
CONCLUSION
The estimated prevalence of familial aneurysm among a Thai population was approximately 9.05%.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bakker MK, van der Spek RA, van Rheenen W, Morel S, Bourcier R, Hostettler IC. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. 2020. 52: 1303-13
2. Bor AS, Koffijberg H, Wermer MJ, Rinkel GJ. Optimal screening strategy for familial intracranial aneurysms a cost-effectiveness analysis. Neurology. 2010. 74: 1671-9
3. Brown RD, Broderick JP. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014. p. 13393-404
4. Chan DY, Abrigo JM, Cheung TC, Siu DY, Poon WS, Ahuja AT. Screening for intracranial aneurysms? Prevalence of unruptured intracranial aneurysms in Hong Kong Chinese. J Neurosurg. 2016. 124: 1245-9
5. Chen X, Liu Y, Tong H, Dong Y, Ma D, Xu L. Meta-analysis of computed tomography angiography versus magnetic resonance angiography for intracranial aneurysm. Medicine (Baltimore). 2018. 97: e10771
6. Gorelick PB. Should we be screening relatives of patients with aneurysmal subarachnoid hemorrhage for asymptomatic intracranial aneurysm?. Curr Atheroscler Rep. 2000. 2: 89-91
7. Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996. 25: 1107-16
8. Harada K, Fukuyama K, Shirouzu T, Ichinose M, Fujimura H, Kakumoto K. Prevalence of unruptured intracranial aneurysms in healthy asymptomatic Japanese adults: Differences in gender and age. Acta Neurochir (Wien). 2013. 155: 2037-43
9. Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008. 40: 217-24
10. Inagawa T, Hirano A. Ruptured intracranial aneurysms: An autopsy study of 133 patients. Surg Neurol. 1990. 33: 117-123
11. Intarakhao P, Thiarawat P, Tewaritrueangsri A, Pojanasupawun S. Low-dose adenosine-induced transient asystole during intracranial aneurysm surgery. Surg Neurol Int. 2020. 11: 235
12. Ishikawa Y, Hirayama T, Nakamura Y, Ikeda K. Incidental cerebral aneurysms in acute stroke patients: Comparison of asymptomatic healthy controls. J Neurol Sci. 2010. 298: 42-5
13. Jakubowski J, Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978. 41: 972-9
14. Jellinger K. Pathology of intracerebral hemorrhage. Zentralbl Neurochir. 1977. 38: 29-42
15. Jiranukool J, Thiarawat P, Galassi W. Prevalence of intracranial aneurysms among acute ischemic stroke patients. Surg Neurol Int. 2020. 11: 341
16. Kirkpatrick PJ, McConnell RS. Screening for familial intracranial aneurysms. BMJ. 1999. 319: 1512-3
17. Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol. 2016. 12: 50-5
18. Mackey J, Brown RD, Moomaw CJ, Sauerbeck L, Hornung R, Gandhi D. Unruptured intracranial aneurysms in the familial intracranial aneurysm and international study of unruptured intracranial aneurysms cohorts: Differences in multiplicity and location clinical article. J Neurosurg. 2012. 117: 60-4
19. Miller TD, White PM, Davenport RJ, Al-Shahi Salman R. Screening patients with a family history of subarachnoid haemorrhage for intracranial aneurysms: Screening uptake, patient characteristics and outcome. J Neurol Neurosurg Psychiatry. 2012. 83: 86-8
20. Oh YS, Lee SJ, Shon YM, Yang DW, Kim BS, Cho AH. Incidental unruptured intracranial aneurysms in patients with acute ischemic stroke. Cerebrovasc Dis. 2008. 26: 650-3
21. Raaymakers TW, Buys PC, Verbeeten B, Ramos LM, Witkamp TD, Hulsmans FJ. MR angiography as a screening tool for intracranial aneurysms: Feasibility, test characteristics, and interobserver agreement. AJR Am J Roentgenol. 1999. 173: 1469-75
22. Rinkel GJ. Intracranial aneurysm screening: Indications and advice for practice. Lancet Neurol. 2005. 4: 122-8
23. Ronkainen A, Hernesniemi J, Kuivaniemi H, Tromp G. Current implications for the efficacy of noninvasive screening for occult intracranial aneurysms in patients with a family history of aneurysms. J Neurosurg. 1996. 84: 534-6
24. Ronkainen A, Puranen MI, Hernesniemi JA, Vanninen RL, Partanen PL, Saari JT. Intracranial aneurysms: MR angiographic screening in 400 asymptomatic individuals with increased familial risk. Radiology. 1995. 195: 35-40
25. Rustemi O, Alaraj A, Shakur SF, Orning JL, Du X, Aletich VA. Detection of unruptured intracranial aneurysms on noninvasive imaging. Is there still a role for digital subtraction angiography? Surg Neurol Int 2015. ;. 6: 175
26. Takahashi E, Haku M, Suzuki Y, Fukagawa K, Iida Y, Yoshida K. Effectiveness of magnetic resonance angiography for mass screening of unruptured intracranial aneurysms. Nihon Koshu Eisei Zasshi. 1997. 44: 509-17
27. Thien A, See AA, Ang SY, Primalani NK, Lim MJ, Ng YP. Prevalence of asymptomatic unruptured intracranial aneurysms in a Southeast Asian population. World Neurosurg. 2017. 97: 326-32
28. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
29. Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: An updated meta-analysis. Stroke. 2007. 38: 1404-10
30. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10