- Departments of Neurosurgery, Fundacion Jimenez Diaz University Hospital, Madrid, Spain.
- Departments of Radiology, Fundacion Jimenez Diaz University Hospital, Madrid, Spain.
- Department of Neurosurgery, University Clinic of Navarra, Pamplona, Navarra, Spain.
Correspondence Address:
Pierre Ferrer
Departments of Neurosurgery, Fundacion Jimenez Diaz University Hospital, Madrid, Spain.
DOI:10.25259/SNI_185_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pierre Ferrer, Pablo Barbero, Gonzalo Monedero, Anna Lo Presti, Bartolome Bejarano, Juan Ramon Penanes. Primary central nervous system lymphoma and 5-aminolevulinic acid. 23-May-2020;11:122
How to cite this URL: Pierre Ferrer, Pablo Barbero, Gonzalo Monedero, Anna Lo Presti, Bartolome Bejarano, Juan Ramon Penanes. Primary central nervous system lymphoma and 5-aminolevulinic acid. 23-May-2020;11:122. Available from: https://surgicalneurologyint.com/surgicalint-articles/10040/
Abstract
Background: Despite surgical resection of primary central nervous system lymphomas (PCNSL) having been always discouraged, recent evidence supports that it might improve prognosis in this patient population. Five- aminolevulinic acid-derived fluorescence is widely used for the resection of malignant gliomas, but its role in PCNSL surgery remains unclear.
Case Description: We present two patients with a solitary solid intraparenchymal mass. As high-grade glioma leaded the list of differential diagnosis (other possibilities were metastasis, abscess, and PCNSL), a five- aminolevulinic acid-guided complete resection (with strong fluorescence in both cases) was done. Surgery was uneventfully carried on with complete resection until five-aminolevulinic acid-induced fluorescence was no longer evident. After surgery, patients have no neurological deficits and had good recovery. Pathological examination revealed that both tumors were PCNSL. Adjuvant radiotherapy and chemotherapy were started. After 1 year of follow-up, patients have good evolution and have no recurrences.
Conclusion: These cases add to the growing literature which shows that surgery might play an important role in the management of PCNSL with an accessible and single lesion. Five-aminolevulinic acid could also be a useful tool to achieve complete resection and improve prognosis in this group of patients.
Keywords: 5-Aminolevulinic acid, Brain injuries, Lymphoma, Neuro-oncology, Neurosurgery
INTRODUCTION
Surgical resection of primary central nervous system lymphoma (PCNSL) is not routinely performed as its conventional management has regularly consisted of radiation treatment and chemotherapy after obtaining pathology diagnosis through biopsy. In addition, the role of 5-aminolevulinic acid (5-ALA)-induced fluorescence which has been established almost as protocol for malignant gliomas resection remains unexplored in PCNSL. Here, we describe two cases of PCNSL treated using 5-ALA-induced fluorescence-guided surgical resection with good clinical and radiological outcome.
CASE DESCRIPTION
We present two cases. They were in their sixties, a man (patient 1) and a woman (patient 2) without any significant medical history, who presented to our emergency department with headache. Neurological examination was normal. Magnetic resonance image (MRI) of the brain demonstrated in both cases a solitary solid intraparenchymal mass in the right frontal lobe with perilesional edema. The lesions were hypointense in noncontrast T1-weighted images and hyperintense in T2-weighted images. After contrast administration, both lesions enhanced with a ring-like pattern [
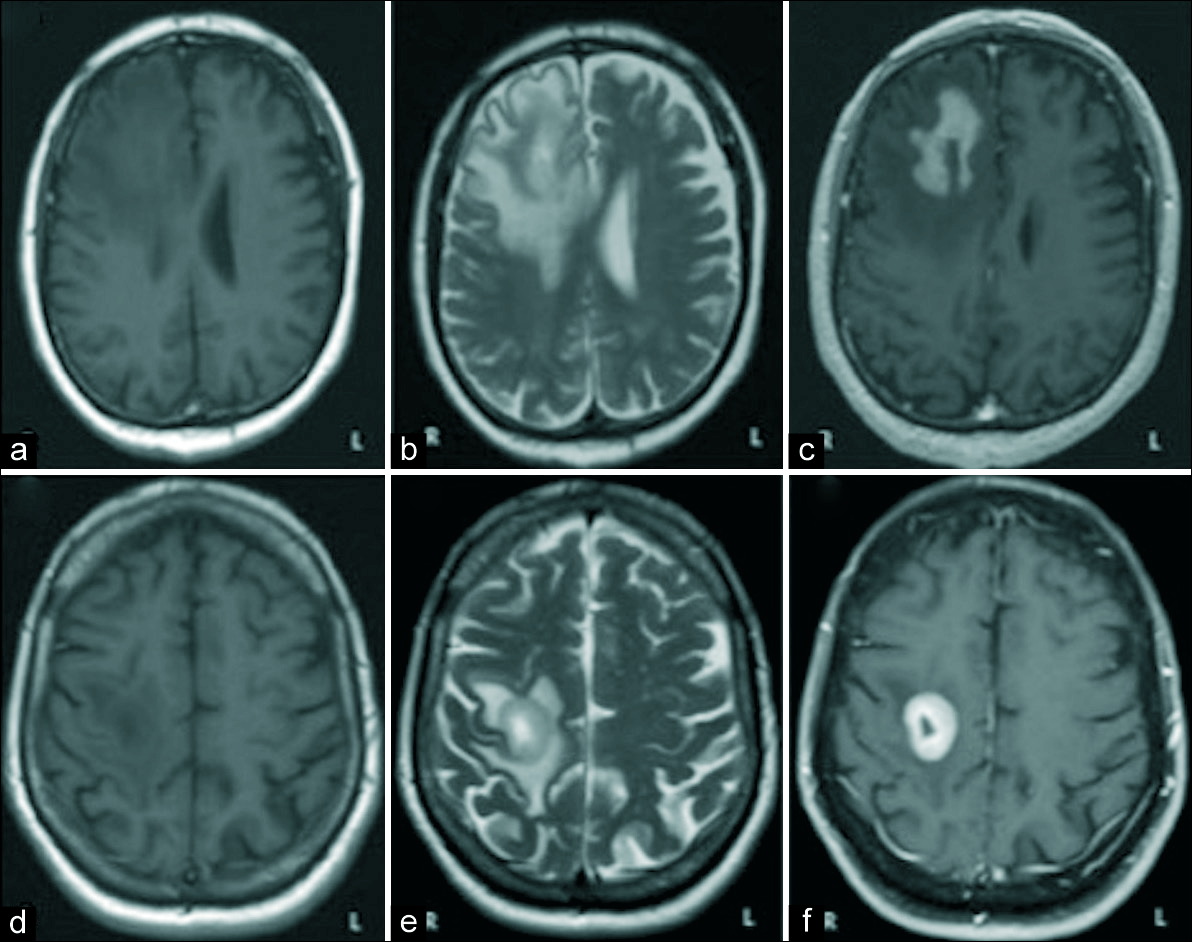
Figure 1:
The images a, b, and c correspond to the patient 1. The images d, e, and f correspond to the patient 2. In both cases, we can appreciate a single intra-axial lesion in the right frontal lobe, prominently hypointense in T1 sequences (a and d) and hyperintense in T2 sequences (b and e). It shows intense contrast enhancement with hypocaptant center of cystic/necrotic aspect and is surrounded by a large halo of edema with vasogenic characteristics (c and f).
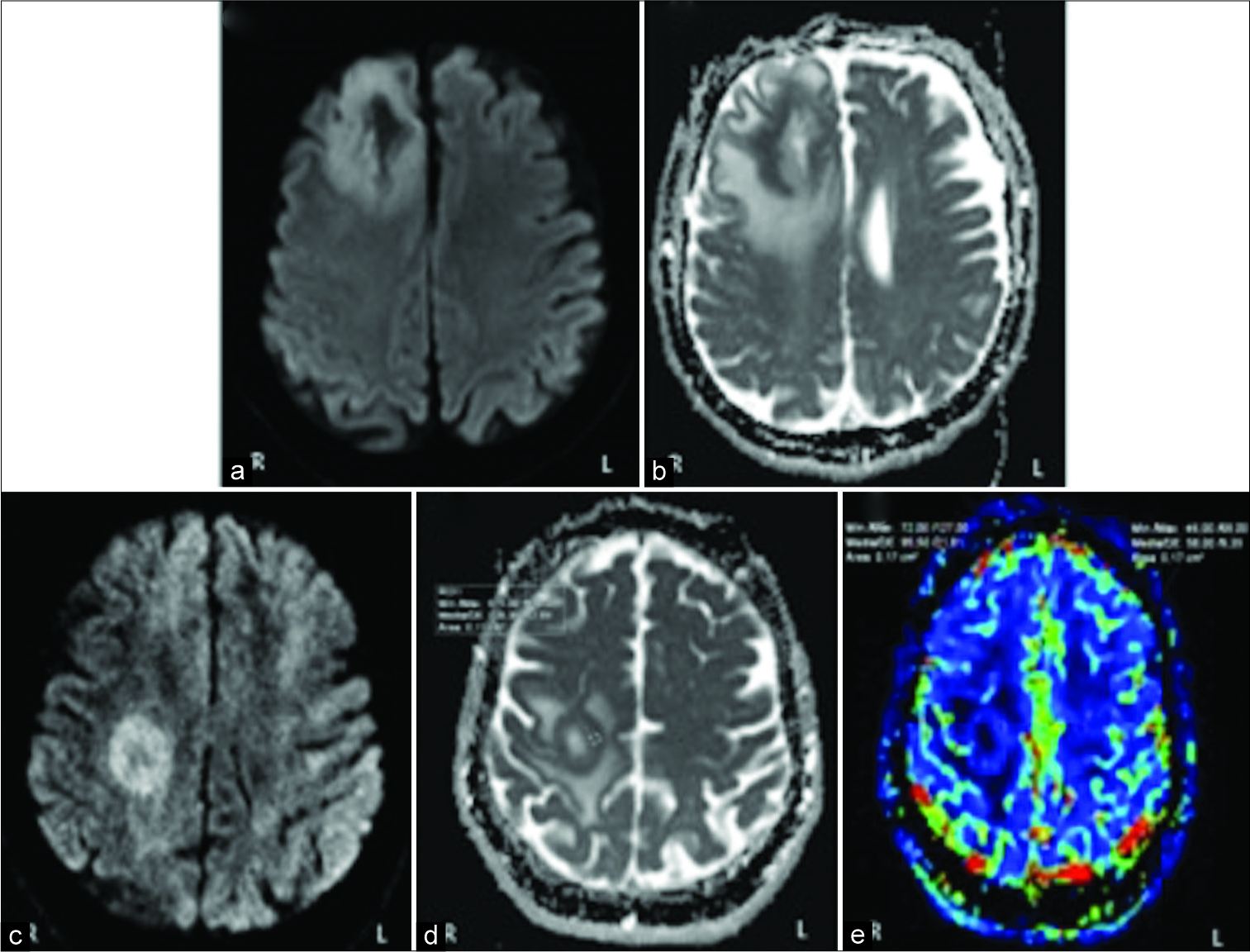
Figure 2:
The images a and b correspond to the patient 1. The images c, d, and e correspond to the patient 2. Diffusion-weighted image demonstrate a clear rim of restricted diffusion in its solid marginal components: periphery of the lesion evince hyperintensity on diffusion- weighted imaging (a and c) and very low values on apparent diffusion coefficient map (b and d). In the perfusion study of the patient 2 (e), the peripheral region of the lesion manifests a slight increase in relative cerebral blood volume about 1.6 respect to the healthy contralateral white matter.
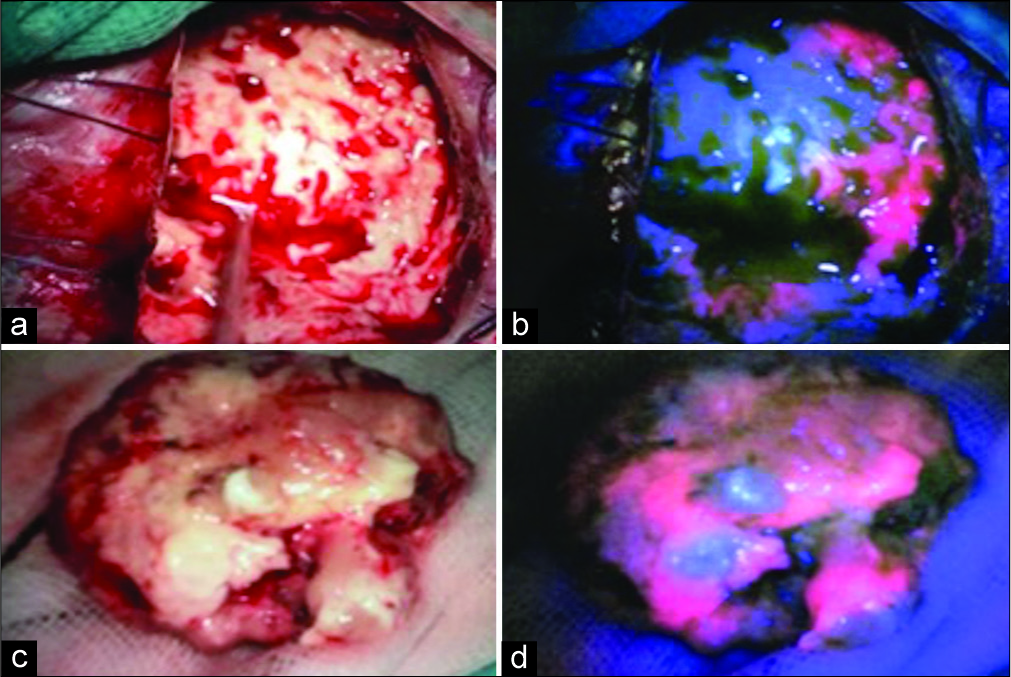
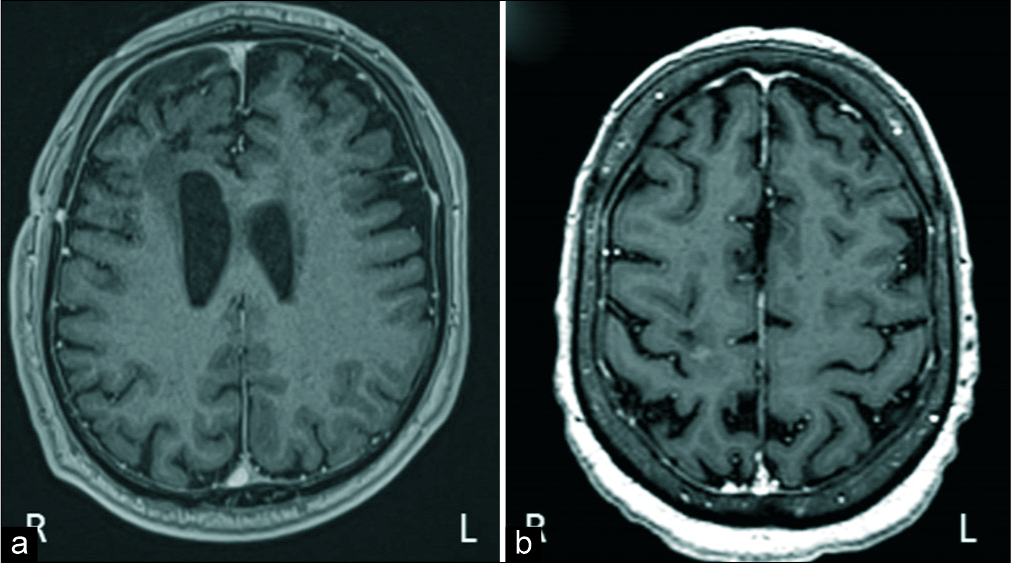
As high-grade glioma leaded the list of differential diagnosis, 5-ALA-guided surgery was recommended. During the intervention, there was strong 5-ALA-induced tumor fluorescence in both cases. Surgery was done with the navigation system StealthStationTM S8 (Medtronic, Minneapolis, USA), and the area of 5-ALA fluorescence corresponds exactly with the gadolinium enhancement on MRI in both cases. Surgery was uneventfully carried on until 5-ALA-induced fluorescence was no longer evident [
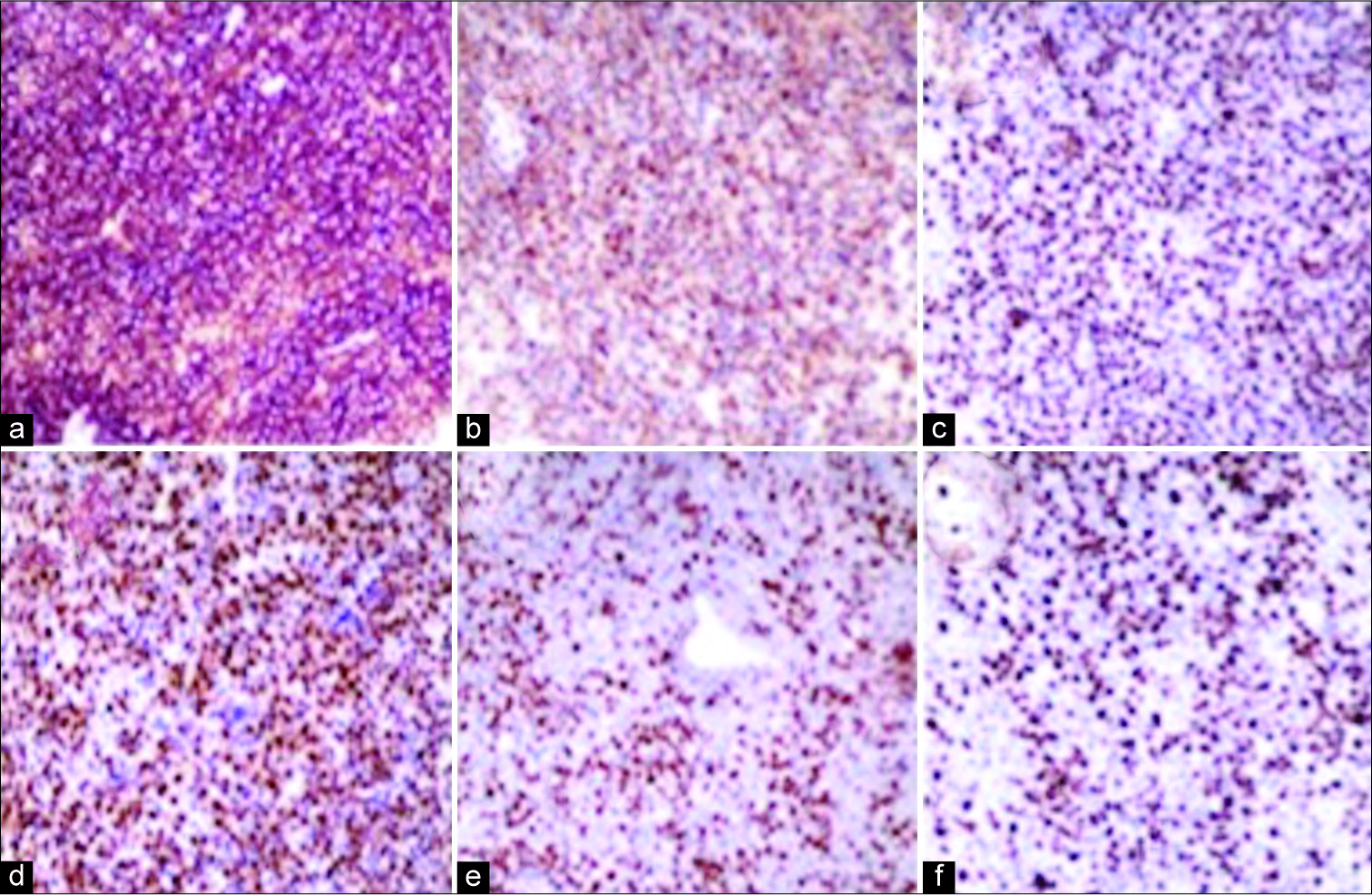
Pathological examination confirms that both tumors were PCNSL. They showed diffuse lymphoid cell proliferation immunoreactive for B-cell marker but not for T-cell marker [
DISCUSSION
PCNSL are rare and aggressive central nervous system (CNS) neoplasms.[
Nowadays, the management of PCNSL consists of biopsy to obtain pathological diagnosis followed by chemotherapy and radiation therapy.[
New advances in surgical technology and adjuvant treatments have led to more aggressive approaches reconsidering the role of surgical resection in PCNSL.[
While the use of 5-ALA is known to be associated with increased extent of resection that leads to improved progression-free survival in patients with high-grade gliomas, its role in PCNSL remains unclear. Yamamoto et al.[
CONCLUSION
PCNSL is rare primary CNS tumors associated with substantial morbidity and mortality. The field of this tumor management is evolving substantially in recent decades and will continue to do so. These cases add to the growing literature which shows that surgery resection is a safe and effective method that might improve overall survival in patients with accessible PCNSL and single lesions, and it could be optimized by the use of 5-ALA. The follow-up period after treatment was 1 year, so long-term results are needed in future to confirm these good outcomes. In this context, there is a need for prospective studies to investigate the safety and therapeutic benefit of cytoreductive surgery for PCNSL.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
No.
Conflicts of interest
There are no conflicts of interest.
References
1. Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M. Primary intracerebral malignant lymphoma: Report of 248 Cases. J Neurosurg. 2000. 92: 261-6
2. Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: A series of 33 cases. Eur J Surg Oncol. 2005. 31: 100-5
3. Caroli E, Acqui M, Ferrante L. Primary cerebral lymphoma: A retrospective study in 22 immunocompetent patients. Tumori. 2004. 90: 294-8
4. Evers G, Kamp M, Warneke N, Berdel W, Sabel M, Stummer W. 5-aminolaevulinic acid-induced fluorescence in primary central nervous system lymphoma. World Neurosurg. 2017. 98: 375-80
5. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: Characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011. 32: 984-92
6. Jelicic J, Todorovic Balint M, Raicevic S, Ilic R, Stanisavljevic D, Bila J. The possible benefit from total tumour resection in primary diffuse large B-cell lymphoma of central nervous system-a one-decade single-centre experience. Br J Neurosurg. 2016. 30: 80-5
7. Lukas RV, Stupp R, Gondi V, Raizer JJ. Primary central nervous system lymphoma-PART 1: Epidemiology, diagnosis, staging, and prognosis. Oncology (Williston Park). 2018. 32: 17-22
8. Lukas RV, Stupp R, Gondi V, Raizer JJ. Primary central nervous system lymphoma-PART 2: Modern therapeutic management and future directions. Oncology (Williston Park). 2018. 32: e11-9
9. Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011. 32: 1004-10
10. Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016. 18: v1-75
11. Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016. 174: 417-24
12. Weller M, Martus P, Roth P, Thiel E, Korfel A, German PCNSL Study Group. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012. 14: 1481-4
13. Yamamoto T, Ishikawa E, Miki S, Sakamoto N, Zaboronok A, Matsuda M. Photodynamic diagnosis using 5-aminolevulinic acid in 41 biopsies for primary central nervous system lymphoma. Photochem Photobiol. 2015. 91: 1452-7
14. Yun J, Iwamoto FM, Sonabend AM. Primary central nervous system lymphoma: A critical review of the role of surgery for resection. Arch Cancer Res. 2016. 4: 71-