- Department of Neurosurgery, Kansai Medical University, Shinmachi, Hirakata, Japan.
Correspondence Address:
Masahiro Nonaka, Department of Neurosurgery, Kansai Medical University, Shinmachi, Hirakata, Japan.
DOI:10.25259/SNI_356_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Katsuya Ueno, Masahiro Nonaka, Tetsuo Hashiba, Yi Li, Takamasa Kamei, Junichi Takeda, Akio Asai. Primary central nervous system lymphoma of the tectal plate in adult. 22-Jul-2022;13:319
How to cite this URL: Katsuya Ueno, Masahiro Nonaka, Tetsuo Hashiba, Yi Li, Takamasa Kamei, Junichi Takeda, Akio Asai. Primary central nervous system lymphoma of the tectal plate in adult. 22-Jul-2022;13:319. Available from: https://surgicalneurologyint.com/surgicalint-articles/11731/
Abstract
Background: Primary central nervous system lymphoma (PCNSL) originating in the brainstem is uncommon. In particular, PCNSL confined to the tectal plate in adults has never been reported in the past. The case of a 53-year-old man who was diagnosed with PCNSL in the tectal plate is reported.
Case Description: The patient was referred to our hospital with a 1-month history of disorientation and magnetic resonance imaging showed hydrocephalus with an enhancing lesion in the tectum. Preoperative blood tests showed a high serum soluble interleukin-2 receptor level of 624 U/ml. Through a single burr hole, endoscopic third ventriculostomy and biopsy of the lesion were simultaneously performed with a flexible endoscope. The histological examination confirmed diffuse large B-cell lymphoma. The patient underwent chemotherapy and radiotherapy.
Conclusion: Malignant lymphoma of the tectum may occur in adults. By measuring the soluble interleukin-2 level preoperatively, it was possible to include malignant lymphoma in the differential diagnosis. In addition, the use of a neuroendoscope permits biopsy and hydrocephalus treatment to be performed simultaneously.
Keywords: Endoscopy, Primary central nervous system lymphoma, Tectum
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) originating in the brainstem has been reported to account for 10% of all PCNSL cases, including multiple lesions.[
CASE PRESENTATION
The patient was a 53-year-old man who had a history of testicular seminoma that was removed twice, at the ages of 41 and 48 years. The patient had been experiencing disorientation and difficulty in thinking for 1 month before the visit. His Karnofsky Performance Status (KPS) at the time of the first visit was 80. Contrast-enhanced magnetic resonance imaging (MRI) showed a markedly contrast-enhanced mass in the dorsal midbrain and obstructive hydrocephalus [
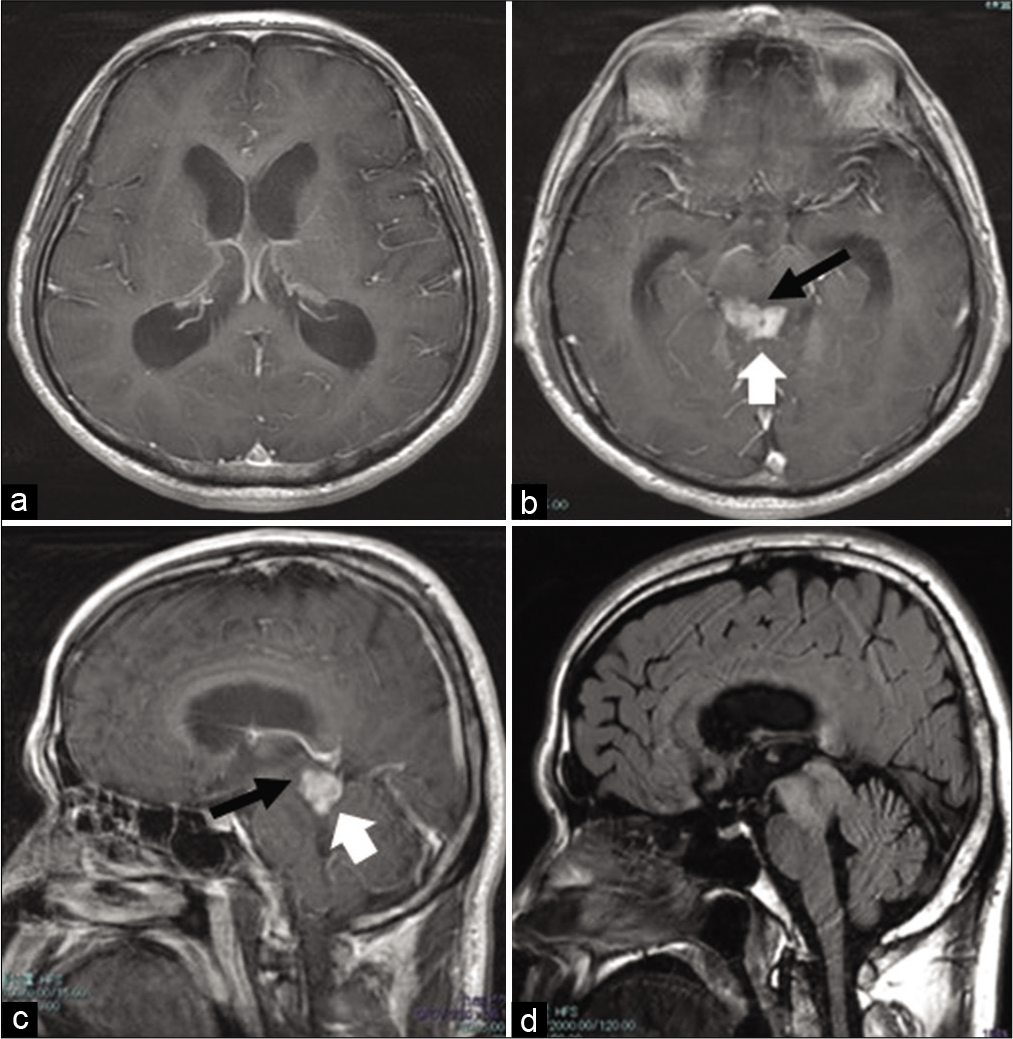
Figure 1:
MR images obtained before surgery. (a and b): Gd-enhanced T1-weighted axial images. (c): Gd-enhanced T1-weighted sagittal images. The size of the tumor is 19 × 13 × 13 mm3, and it is present in the tectum (white arrow, b, c ). The aqueduct can be seen on the ventral side of the tumor (black arrow, b,c). Both inferior horns of the lateral ventricle are enlarged. (d):FLAIR sagittal images. A FLAIR high-signal area is observed in the entire midbrain surrounding the Gd-enhanced lesion.
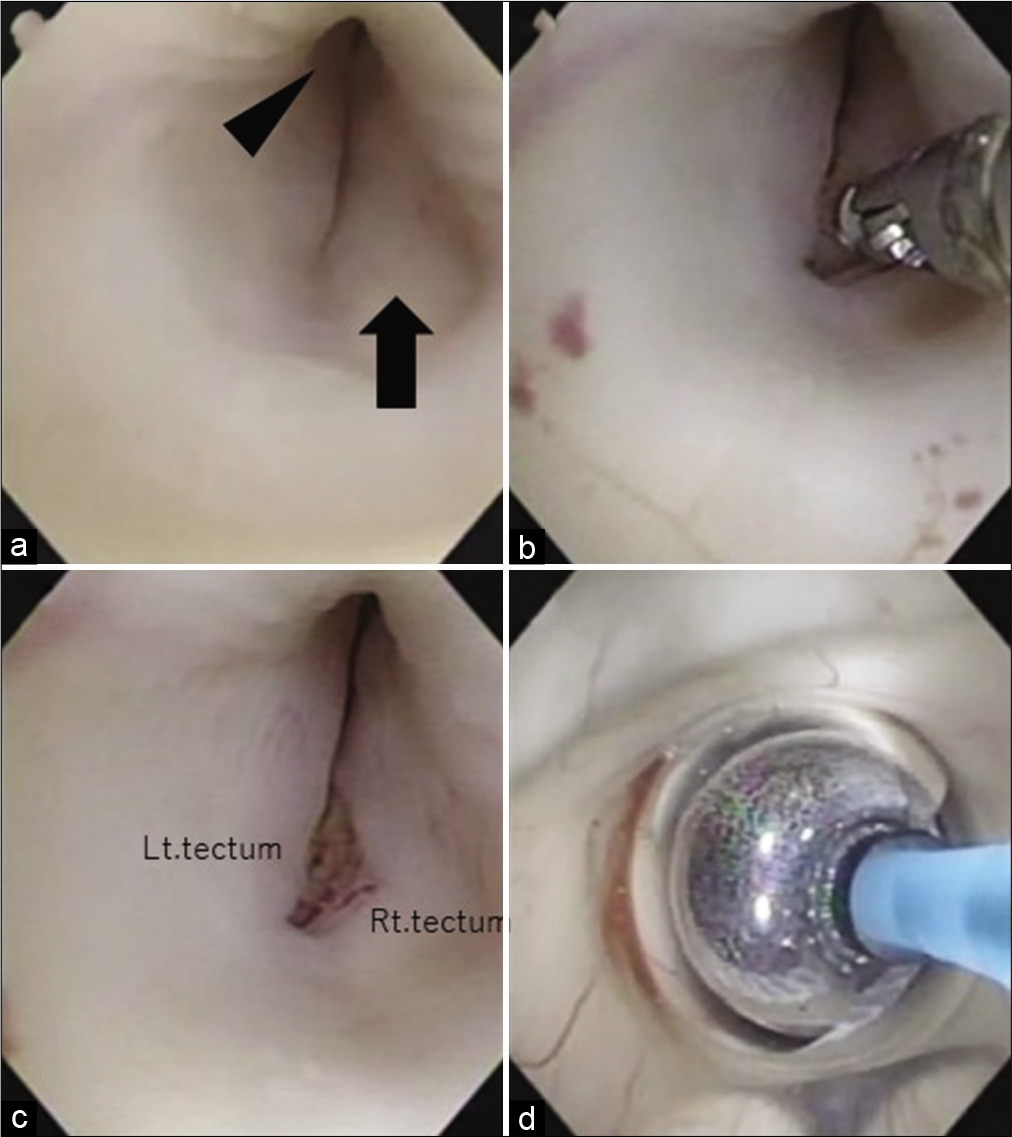
Figure 2:
Intraoperative findings. (a) Swollen tectal plate (black arrow) with aqueductal stenosis (arrowhead). (b and c) Partial removal of the abnormal lesion using a flexible endoscope and biopsy forceps. (d) After the biopsy of the lesion is completed, a third ventriculostomy is performed. In this picture, the floor of the third ventricle is expanded with a balloon.
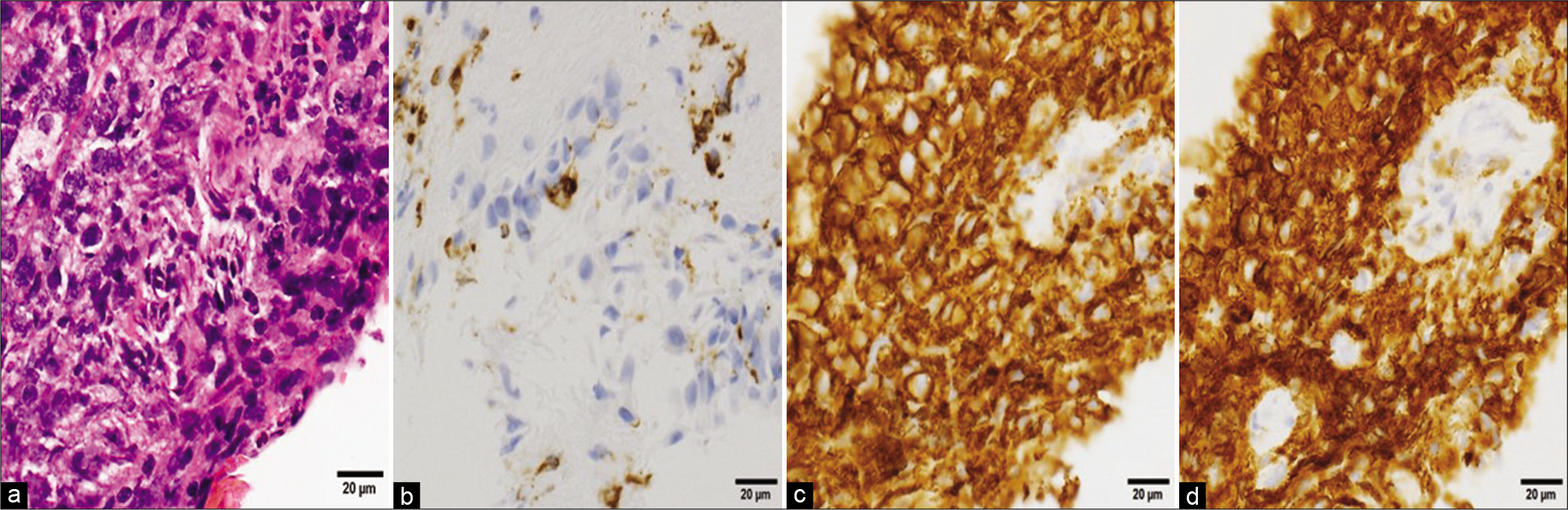
Figure 3:
Histopathological findings of the tumor. (a) Hematoxylin and eosin staining (×40) shows dense proliferation of bare nucleated cells with enlarged nuclei and increased chromatin density. (b-d) The tumor cells are CD3 negative (b), CD20 positive (c), and CD79a positive (d) on immunohistochemistry.
DISCUSSION
PCNSL accounts for 2.4–3% of all brain tumors and 4–6% of all extranodal lymphomas.[
To the best of our knowledge, only one case of PCNSL in the tectum has been reported in a child and the current report is the first adult case.[
In the previous pediatric case, the pathological diagnosis was made by total resection of the tumor by craniotomy.[
In this study, tumor biopsy and ETV were performed at the same time using a flexible endoscope because it causes less brain retraction than a rigid endoscope, which allows a single trajectory to perform a biopsy and ETV.[
The periaqueductal gray matter is thought to contain the centers of the ascending reticular activating system, which lies mainly on the ventral side of the aqueduct. Fortunately, since the tumor was located in the tectum, the dorsal part of the aqueduct, it was possible to biopsy the tumor relatively safely. After removing the ependyma, the brain parenchyma was not sampled deeply so as avoid causing damage to the superior and inferior colliculi. The use of endoscopes is highly beneficial for patients because it allows them to quickly move on to chemotherapy and other treatments without complications and with as little invasion as possible.
Extracranial malignant lymphomas have been reported to develop as second malignancies after radiation or chemotherapy for pure seminoma.[
CONCLUSION
To the best of our knowledge, this is the first case report of PCNSL occurring in the tectum of an adult, and biopsy and ETV of the tectal lesion were safely performed using a flexible endoscope. The preoperative serum soluble interleukin-2 receptor level was helpful in the differential diagnosis of this lesion.
Ethical approval and informed consent
This study was approved by the Ethics Committee of Kansai Medical University (No. 2020055). Need for written patient consent was waived by the ethics committee because data were deidentified.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
1. Alimehmeti R, Campanella R, Bauer D, Balbi S, Rampini P, Egidi M. Intracranial metastasis of testicular seminoma in an HIV-positive. Case report and review. J Neurooncol. 2003. 65: 135-40
2. Benson R, Mallick S, Purkait S, Suri V, Haresh KP, Gupta S. Primary pediatric mid-brain lymphoma: Report of a rare pediatric tumor in a rare location. World J Clin Cases. 2016. 4: 419-22
3. Bognar L, Turjman F, Villanyi E, Mottolese C, Guyotat J, Fischer C. Tectal plate gliomas. Part II: CT scans and MR imaging of tectal gliomas. Acta Neurochir (Wien). 1994. 127: 48-54
4. Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004. 28: 464-70
5. Dedushi K, Kabashi S, Ugurel MS, Ramadani N, Mucaj S, Zeqiraj K. Magnetic resonance imaging of a case of central neurocytoma. Acta Inform Med. 2016. 24: 419-21
6. Ishikawa T, Takeuchi K, Tsukamoto N, Kawabata T, Wakabayashi T. A novel dissection method using a flexible neuroendoscope for resection of tumors around the aqueduct of sylvius. World Neurosurg. 2018. 110: 391-6
7. Javadpour M, Mallucci C. The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst. 2004. 20: 852-7
8. Kon T, Kakita A, Koide A, Mori H, Tanaka R, Takahashi H. A primary CNS lymphoma in spontaneous remission for 3.5 years after initial detection of the lesions by MRI. Brain Tumor Pathol. 2003. 20: 27-31
9. Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: A whole-brain disease?. Neurology. 2002. 59: 1557-62
10. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Ruther U, Dieckmann KP, Bussar-Maatz R, Eisenberger F. Second malignancies following pure seminoma. Oncology. 2000. 58: 75-82
12. Sato S, Shibahara I, Inoue Y, Hide T, Kumabe T. New radiologic findings of hypertrophic olivary degeneration in 2 patients with brainstem lymphoma. World Neurosurg. 2019. 123: 464-8.e461
13. Schlegel U. Primary CNS lymphoma. Ther Adv Neurol Disord. 2009. 2: 93-104
14. Tarulli AW, Lim C, Bui JD, Saper CB, Alexander MP. Central neurogenic hyperventilation: A case report and discussion of pathophysiology. Arch Neurol. 2005. 62: 1632-4
15. Ueno K, Nonaka M, Isozaki H, Kamei T, Takeda J, Asai A. Resection of a recurrent medulloblastoma in the anterior middle part of the aqueduct with a flexible endoscope: A case report. Childs Nerv Syst. 2021. 37: 665-9