- Department of Neurosurgery, Staten Island University Hospital, Staten Island, New York, United States,
- Department of Neurology, Staten Island University Hospital, Staten Island, New York, United States,
- Department of Hematology/Oncology, Staten Island University Hospital, Staten Island, New York, United States.
Correspondence Address:
Sabastian Hajtovic, Department of Neurosurgery, Staten Island University Hospital, Staten Island, New York, United States.
DOI:10.25259/SNI_54_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sabastian Hajtovic1, Edward Yu2, Alexander Bershadskiy3, Raphael Sacho1, Ronit Gilad1. Primary intracranial marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the lateral ventricle: Case report and review of pathogenesis. 29-Apr-2022;13:181
How to cite this URL: Sabastian Hajtovic1, Edward Yu2, Alexander Bershadskiy3, Raphael Sacho1, Ronit Gilad1. Primary intracranial marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the lateral ventricle: Case report and review of pathogenesis. 29-Apr-2022;13:181. Available from: https://surgicalneurologyint.com/surgicalint-articles/11563/
Abstract
Background: Primary central nervous system lymphoma (PCNSL) is an aggressive extranodal subtype of nonHodgkin’s lymphoma. Ventricle-predominant PCNSL, arising in the CNS ventricular system, is a rare entity. In over 90% of cases, PCNSL is classified as diffuse large B-cell lymphoma. Rarely, PCNSL may be classified as marginal zone B-cell lymphoma (MZBCL) of mucosa-associated lymphoid tissue (MALT). Taken together, a primary MALT-type MZBCL arising in a cerebral ventricle is an extremely rare presentation.
Case Description: A 69-year-old female presented with a persistent left frontal headache for 1 year. Magnetic resonance imaging revealed an enhancing soft-tissue lesion within the left lateral ventricle, with associated periventricular edema. We performed an excisional biopsy of the tumor, which grossly had the appearance of a meningioma. Histopathology of the tumor was consistent with MZBCL of the MALT type. The patient was treated with Rituximab and Ibrutinib. Six months after surgery, she remained neurologically intact and free of disease.
Conclusion: We report the case of a primary MALT-type MZBCL arising in the CNS ventricular system, with characteristics mimicking meningioma. This lymphoma involved the lateral ventricle and likely originated from the choroid plexus. Meningothelial cells and epithelial cells in the choroid plexus may acquire MALT in response to chronic inflammatory stimuli, such as infection or autoimmune disease. In rare cases, MALT lymphoma may develop as part of this pathogenesis.
Keywords: Cerebral ventricle, Choroid plexus, CNS lymphoma, MALT lymphoma, Marginal zone B-cell lymphoma
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is an aggressive extranodal subtype of non-Hodgkin lymphoma (NHL) arising in the brain, spinal cord, eyes, or leptomeninges.[
PCNSL is classified as diffuse large B-cell lymphoma in 90% of cases.[
Primary MALT-type MZBCL arising in an intraventricular location of the CNS is an extremely rare presentation. To the best of our knowledge, this has only been reported in three cases.[
CASE PRESENTATION
A 69-year-old right-handed female with a medical history of migraines presented with a persistent left frontal headache for 1 year. She characterized the headache as morning onset, non-radiating, and lasting several minutes with spontaneous resolution. It was not associated with visual changes, nausea, vomiting, altered mental status, or constitutional symptoms. Neurologic examination showed no focal deficits.
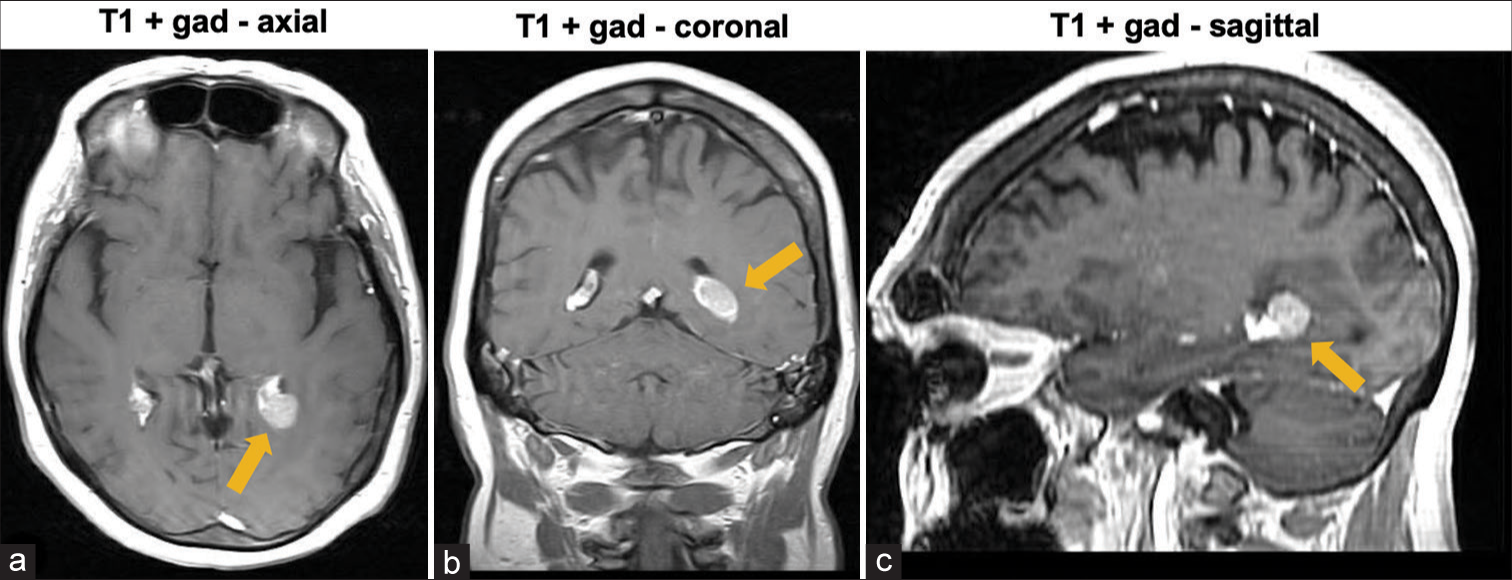
Magnetic resonance imaging (MRI) revealed an enhancing soft-tissue lesion within the atrium of the left lateral ventricle [
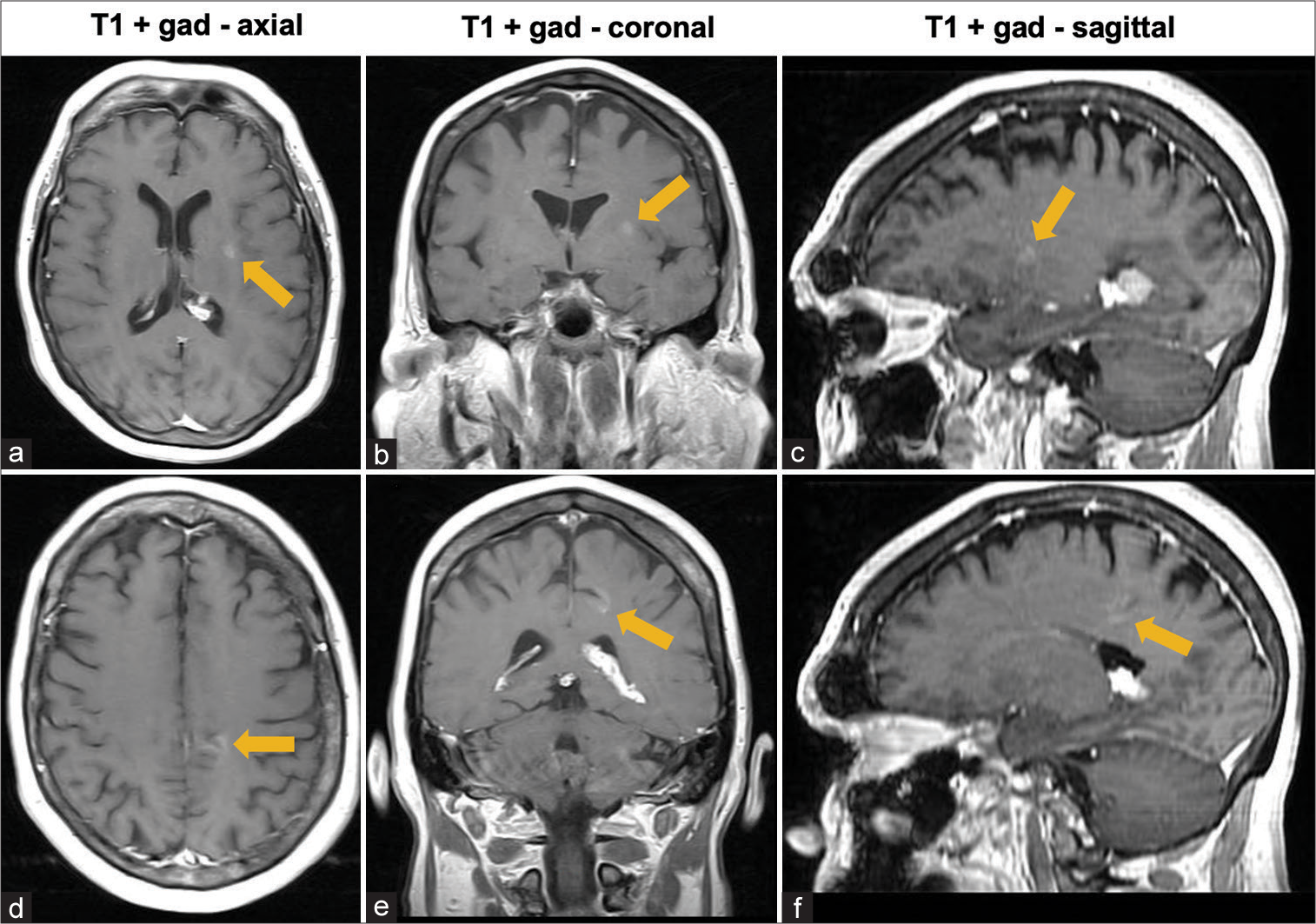
Five months earlier, the patient presented to the hospital with similar complaints. Complete workup, including computed tomography (CT) scan of the chest, abdomen, and pelvis, and lumbar puncture to evaluate for infectious, inflammatory, and neoplastic causes, was negative. MRI showed the same intraventricular lesion coinciding with the choroid plexus, in addition to the two enhancing lesions in the parenchyma. However, at this time, postinfectious inflammation from COVID-19 infection 4 months before the onset of headaches was considered a potential etiology of the lesions and symptoms. Thus, no treatment was initiated, and surgery was not considered, at this time. Follow-up MRI 5 months later [
We decided to perform an excisional biopsy of the tumor under general anesthesia, with the intent of using direct vision to control potential intraventricular bleeding. A 3 cm × 3 cm left parietal craniotomy was performed. A tubular retractor was guided to the left atrium of the lateral ventricle using neuronavigation. An operating microscope was used for visualization. Once the ventricle was entered, the lesion was firm and encapsulated, in a distinct plane relative to surrounding structures. It was separated from the choroid plexus and removed en bloc to avoid spilling its contents into the ventricular system. Grossly, the tumor had the appearance of a meningioma.
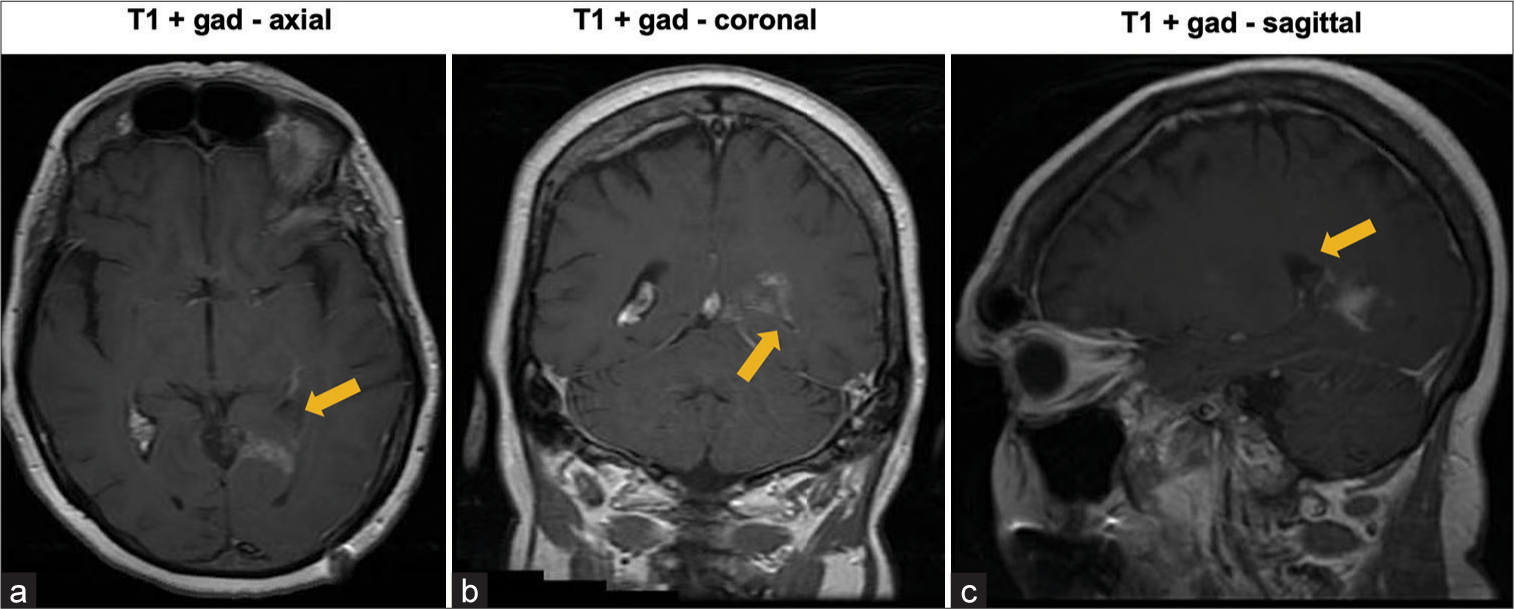
Postoperatively, systemic steroid therapy was initiated. MRI on postoperative day 9 demonstrated gross total resection (GTR) of the tumor [
Pathology of the tumor was consistent with MZBCL of the MALT type. Histologic examination revealed a dense diffuse infiltrate of small lymphoid cells with dark nuclei and scant to moderate amounts of pale cytoplasm, with occasional aggregates resembling germinal centers. Scattered psammomatous calcification was seen. Most tumor cells were CD20 and PAX5 positive B-cells negative for cyclin D1, CD5, CD10, CD43, and MYC. A subset of B-cells was BCL6 and Ki-67 positive in irregular aggregates, consistent with germinal centers. Kappa light chain restriction was present. Tumor cells were negative for Epstein-Barr virus-encoded small RNAs by in situ hybridization. The Ki-67 proliferation index outside germinal centers was 20%.
Additional workup was performed to rule out peripheral involvement. Bone marrow biopsy demonstrated involvement by CD10 and CD5 negative B-cell lymphoma in 10–20% of cells by immunostaining, consistent with the patient’s MZBCL. Fluorodeoxyglucose (FDG) positron emission tomography scan showed no evidence of pathologic FDG uptake.
She is receiving adjuvant treatment with Rituximab, planned for 9 months, and Ibrutinib maintenance therapy planned for 2 years. At 6 months after surgery, she was neurologically intact and free of disease, demonstrated by lumbar puncture and complete neuraxis MRI.
DISCUSSION
We present a rare case of PCNSL of the MALT type of MZBCL originating in the left lateral ventricle of a 69-year-old woman. To the best of our knowledge, only three other cases have been reported in the literature.[
PCNSLs of the MALT type, not limited to intraventricular locations, most commonly present in middle-aged or older women with seizures, headaches, or visual disturbances.[
In a recent review encompassing 45 cases, ventricle-predominant PCNSL (VP-PCNSL) was defined as occurring almost exclusively within the CNS ventricular system, with little or no adjacent parenchymal involvement and no separate intraparenchymal lesions.[
VP-PCNSLs present with a median age of 60.5 years, rapid clinical progression, multifocal disease, edema in adjacent brain tissue, and avid enhancement and restricted diffusion on MRI.[
VP-PCNSLs involve the lateral ventricles in 56% of cases,[
Few plausible hypotheses exist regarding the potential pathogenesis of MALT lymphomas in the CNS, which contains no native mucosal tissue. In one hypothesis, meningothelial cells (MECs) of the CNS serve as a substitute for a mucosal surface.[
Psammoma bodies present in MALT-lymphomas may be an indicator of the presence of meningothelial cells.[
While MECs may serve as a substitute for a mucosal surface, allowing MALT PCNSLs to grow, recruitment of peripheral B lymphocytes to the site of MECs may be an important part of the pathogenesis.[
Infections such as Helicobacter pylori, Borrelia burgdorferi, Campylobacter jejuni, and Chlamydia psittaci, and autoimmune diseases such as Hashimoto’s disease and Sjogren’s syndrome, have been implicated in CNS MZBCLs.[
The standard of care in PCNSL treatment includes high dose methotrexate and rituximab as first-line induction therapy.[
CONCLUSION
We report the case of a primary MALT-type MZBCL arising in the CNS ventricular system, with characteristics mimicking meningioma. This lymphoma involved the lateral ventricle and likely originated from the choroid plexus. Meningothelial cells and epithelial cells in the choroid plexus may acquire MALT in response to chronic inflammatory stimuli, such as infection or autoimmune disease. In rare cases, MALT lymphoma may develop as part of this pathogenesis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ball MK, Morris JM, Wood AJ, Meyer FB, Kaszuba MC, Raghunathan A. Ventricle-predominant primary CNS lymphomas: Clinical, radiological and pathological evaluation of five cases and review of the literature. Brain Tumor Pathol. 2020. 37: 22-30
2. Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M. Primary intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg. 2000. 92: 261-6
3. Estevez M, Chu C, Pless M. Small B-cell lymphoma presenting as diffuse dural thickening with cranial neuropathies. J Neurooncol. 2002. 59: 243-7
4. Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJ, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019. 21: 296-305
5. Hemion C, Li J, Kohler C, Scholl HP, Meyer P, Killer HE. Clearance of neurotoxic peptides and proteins by meningothelial cells. Exp Cell Res. 2020. 396: 112322
6. Itoh T, Shimizu M, Kitami K, Kamata K, Mitsumori K, Fujita M. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the CNS. Neuropathology. 2001. 21: 174-80
7. Jung TY, Jung S, Lee MC, Lee KH. Extranodal marginal zone B-cell lymphoma mimicking meningioma in lateral ventricle: A case report and possible pathogenesis. J Neurooncol. 2006. 80: 63-7
8. Kelley TW, Prayson RA, Barnett GH, Stevens GH, Cook JR, Hsi ED. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the lateral ventricle. Leuk Lymphoma. 2005. 46: 1423-7
9. Le Gars D, Lejeune JP, Peltier J. Surgical anatomy and surgical approaches to the lateral ventricles. Adv Tech Stand Neurosurg. 2009. 34: 147-87
10. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020. 27: 951-61.e5
12. Sanjeevi A, Krishnan J, Bailey PR, Catlett J. Extranodal marginal zone B-cell lymphoma of malt type involving the cavernous sinus. Leuk Lymphoma. 2001. 42: 1133-7
13. Sebastian C, Vela AC, Figueroa R, Marin MA, Alfaro J. Primary intracranial mucosa-associated lymphoid tissue lymphoma. A report of two cases and literature review. Neuroradiol J. 2014. 27: 425-30
14. Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: Biology and physiopathology. J Neuropathol Exp Neurol. 2000. 59: 561-74
15. Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O’Neill BP. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: A primary lowgrade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005. 23: 5718-27