- Department of Biomedical Engineering, Inventum Bioengineering Technologies, LLC, Chicago, Illinois, USA
Correspondence Address:
Kiratipath Iamsakul
Department of Biomedical Engineering, Inventum Bioengineering Technologies, LLC, Chicago, Illinois, USA
DOI:10.4103/sni.sni_371_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kiratipath Iamsakul, Alexander V. Pavlovcik, Jesus I. Calderon, Lance M. Sanderson. PROJECT HEAVEN: Preoperative Training in Virtual Reality. 26-Apr-2017;8:59
How to cite this URL: Kiratipath Iamsakul, Alexander V. Pavlovcik, Jesus I. Calderon, Lance M. Sanderson. PROJECT HEAVEN: Preoperative Training in Virtual Reality. 26-Apr-2017;8:59. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8374
Abstract
A cephalosomatic anastomosis (CSA; also called HEAVEN: head anastomosis venture) has been proposed as an option for patients with neurological impairments, such as spinal cord injury (SCI), and terminal medical illnesses, for which medicine is currently powerless. Protocols to prepare a patient for life after CSA do not currently exist. However, methods used in conventional neurorehabilitation can be used as a reference for developing preparatory training. Studies on virtual reality (VR) technologies have documented VR's ability to enhance rehabilitation and improve the quality of recovery in patients with neurological disabilities. VR-augmented rehabilitation resulted in increased motivation towards performing functional training and improved the biopsychosocial state of patients. In addition, VR experiences coupled with haptic feedback promote neuroplasticity, resulting in the recovery of motor functions in neurologically-impaired individuals. To prepare the recipient psychologically for life after CSA, the development of VR experiences paired with haptic feedback is proposed. This proposal aims to innovate techniques in conventional neurorehabilitation to implement preoperative psychological training for the recipient of HEAVEN. Recipient's familiarity to body movements will prevent unexpected psychological reactions from occurring after the HEAVEN procedure.
Keywords: GEMINI, haptic feedback, HEAVEN, neurorehabilitation, virtual reality
INTRODUCTION
Neurological impairments, such as spinal cord injuries (SCI), resulting in the deterioration of an individual's motor and sensory functions, are currently incurable. Terminal illnesses, in which patient survival is unlikely, have no means of remediation. A cephalosomatic anastomosis (also known as CSA, HEAVEN, head anastomosis venture) is proposed as a last-resort solution to these complications.[
One possible complication to consider in the context of a CSA is the psychological state of the recipient while acclimating to the transplanted body. Because the recipient of HEAVEN is an individual who has complications with motor controls, complete bodily freedom, especially in a foreign, transplanted body, will be an unfamiliar sensation. It is imperative to prevent psychological reactions, e.g., hypomania, stemming from the ability to move normally in a new body. To prepare the recipient for this new normalcy, development of virtual reality (VR) experiences supplemented with haptic feedback is proposed. This method will allow the recipient to experience the sensations of normal bodily movements and reduce the possibility of being psychologically and emotionally overwhelmed after transferring into the new body.
The concepts involved in VR training with haptic feedback are beneficial for rehabilitating patients with neurological impairments. However, the goal of this proposal is specifically for preparation before the HEAVEN procedure. The objective is to utilize and evolve contemporary methods in neurorehabilitation for familiarizing the recipient of HEAVEN to the sensations of performing bodily functions. Familiarity to bodily movement will lower the possibility of the recipient expressing unexpected psychological reactions after the HEAVEN procedure.
PREOPERATIVE VIRTUAL REALITY TRAINING TO PREVENT UNEXPECTED PSYCHOLOGICAL REACTIONS
Once the recipient is able to perform movements in the transplanted body, there is the possibility of being psychologically overwhelmed by the sense of bodily freedom. This might trigger, for instance, hypomania, a dysfunctional condition that can transition into depression, mania, and psychosis.[
This proposal aims to prepare the recipient for the sensations of performing bodily functions and prevent triggering the behaviors mentioned above. Replicating natural body movements through VR experiences will serve as training against unexpected psychological reactions. We speculate that VR training with haptic feedback prior to CSA will sufficiently prepare the recipient for handling the new body and prevent risk-taking behavior caused by psychotic episodes. This approach will require the creation of training that realistically replicates the sensations involved in performing voluntary motor functions. The development of appropriate activities for this approach can be accomplished by referring to the techniques used in conventional neurological rehabilitation.
Overview of virtual reality in neurorehabilitation therapy
Although a standard protocol to prepare the recipient of HEAVEN for life after CSA does not currently exist, conventional approaches to rehabilitation for neurological injuries, such as SCI and stroke, can be used as a reference for developing VR experiences that will assist the recipient in becoming physically, mentally, and emotionally prepared for the unfamiliar normality.
For SCI and stroke rehabilitation, functional training is the most effective approach in promoting neuroplasticity for recovering motor functions. Functional training is a classification of exercises that are used to practice activities of daily living (ADL). This training is used in rehabilitation for the purpose of restoring motor functions such as walking, reaching, and grasping movements.[
Advancements in VR technologies have opened new frontiers for patient care, in particular, enhancing the rehabilitation therapy experience and improving the results of motor recovery in patients with neurological disabilities. There exists several types of VR environments, such as non-immersive, semi-immersive, and immersive VR.[
Rehabilitation supplemented by immersive VR is an approach that has recently been explored in clinical settings, and studies have shown its advantages in improving the quality of recovery in SCI and stroke patients. VR is used as an enhancement to conventional therapy for patients with conditions ranging from musculoskeletal problems and stroke-induced paralysis to cognitive deficits.[
Recent studies on SCI and stroke rehabilitation have noted the benefits that VR and haptic feedback have provided in assisting neurorehabilitation.[
Overview of motivation and biopsychosocial status of patients during virtual reality-augmented rehabilitation
Because VR experiences are generated using computer software (3D rendering software and game engines, etc.), an infinite number of scenarios, activities, and environments can be imagined and created. This advantage allows the creation of customized VR experiences that are suitable for the different learning styles of patients. When designing VR exercises for motor rehabilitation, the major points to consider are the preferences and expectations of the patients.[
Studies on VR-augmented rehabilitation in the clinical setting have shown that patient motivation and time spent performing exercises increased as a result of engaging with VR experiences. When compared to conventional rehabilitation, VR-augmented rehabilitation yields higher motivation during execution of assigned tasks due to the entertaining aspects of VR.[
In addition to improving patient motivation, VR-augmented rehabilitation has been noted to have effects on the biopsychosocial status of patients.[
Overview of the effects of virtual reality and haptic feedback on neuroplasticity
In addition to psychological benefits, VR offers improvements in motor functions as well. Several studies have documented the impact of VR-augmented rehabilitation in improving motor functions of neurologically-impaired individuals. Corbetta et al. conducted a systematic review comparing the effects of VR-augmented rehabilitation on gait, mobility, and balance versus conventional rehabilitation therapy. The review investigated the results from 15 trials involving 341 participants who were clinically diagnosed with stroke. The review reported that VR training provided greater benefits in walking speed, balance, and mobility compared to non-VR rehabilitation.[
VR by itself offers the aforementioned benefits for patients during the rehabilitation process; however, when supplemented with haptic feedback (kinesthetic or tactile), it becomes capable of promoting neuroplasticity in addition to restoring motor functions. Neuroplasticity is defined as the ability of the nervous system to reorganize its structure, function, and connections in response to intrinsic or extrinsic stimuli,[
Providing multisensory training requires the use of VR and haptic feedback. Effectiveness of these two technologies in promoting neuroplasticity has been documented in the study by Donati et al. This study combined immersive VR training, visual-tactile feedback, and walking with EEG-controlled robotic actuators, including a custom-designed lower limb exoskeleton capable of delivering tactile feedback to patients. Chronic SCI paraplegics (n = 8) participated in a 12-month long multistage BMI-based gait neurorehabilitation for the purpose of restoring locomotion. The training resulted in all patients experiencing improvements in somatic sensation, voluntary motor control, walking index, and half of the patients (n = 4) upgraded to an incomplete paraplegia classification. This study suggested that the implementation of tactile feedback played a key role in patient recovery and enhanced the ability of patients to exhibit plasticity during training.[
VR experiences with haptic feedback enables significant and relevant stimulation to the patient's CNS and promotes neuroplasticity. Combining VR experiences with haptic feedback realistically replicates the sensations involved in bodily movement and promotes neuroplastic recovery. Haptic feedback provides stimulation that facilitates the physiological activation of areas in the brain devoted to motor relearning.[
The combination of VR and haptic feedback will provide the best method for promoting neuroplasticity. In preparation for life after HEAVEN, having the recipient train with the combination of these technologies will provide the most realistic imitation of the sensations associated with normal body movement. Although the aim of this proposal is not for the purpose of rehabilitation, but rather preparation, promoting neuroplasticity will serve as a guideline and benchmark for successfully replicating natural body movements.
PROPOSED METHODOLOGY
Preoperative VR training for the recipient will take place several months before the HEAVEN procedure commences. The methodology for the training was formulated in response to the following points:
Referring to exercises in conventional neurorehabilitation, what VR technologies and experiences will be useful for functional training? To provide the most realistic experience, which technologies will provide the most natural feedback in VR in response to an action performed by the user? Sufficiently familiarizing the user with bodily movement is crucial. Will the user remain engaged enough to spend an adequate amount of time training repetitive tasks in VR for three months? How will the user, a neurologically impaired individual, perform activities comfortably without causing pain or overstressing the body? How will progress be measured to ensure that the user is prepared for a new body?
The following solutions are proposed for resolving the points stated above:
Virtual reality technologies and experiences
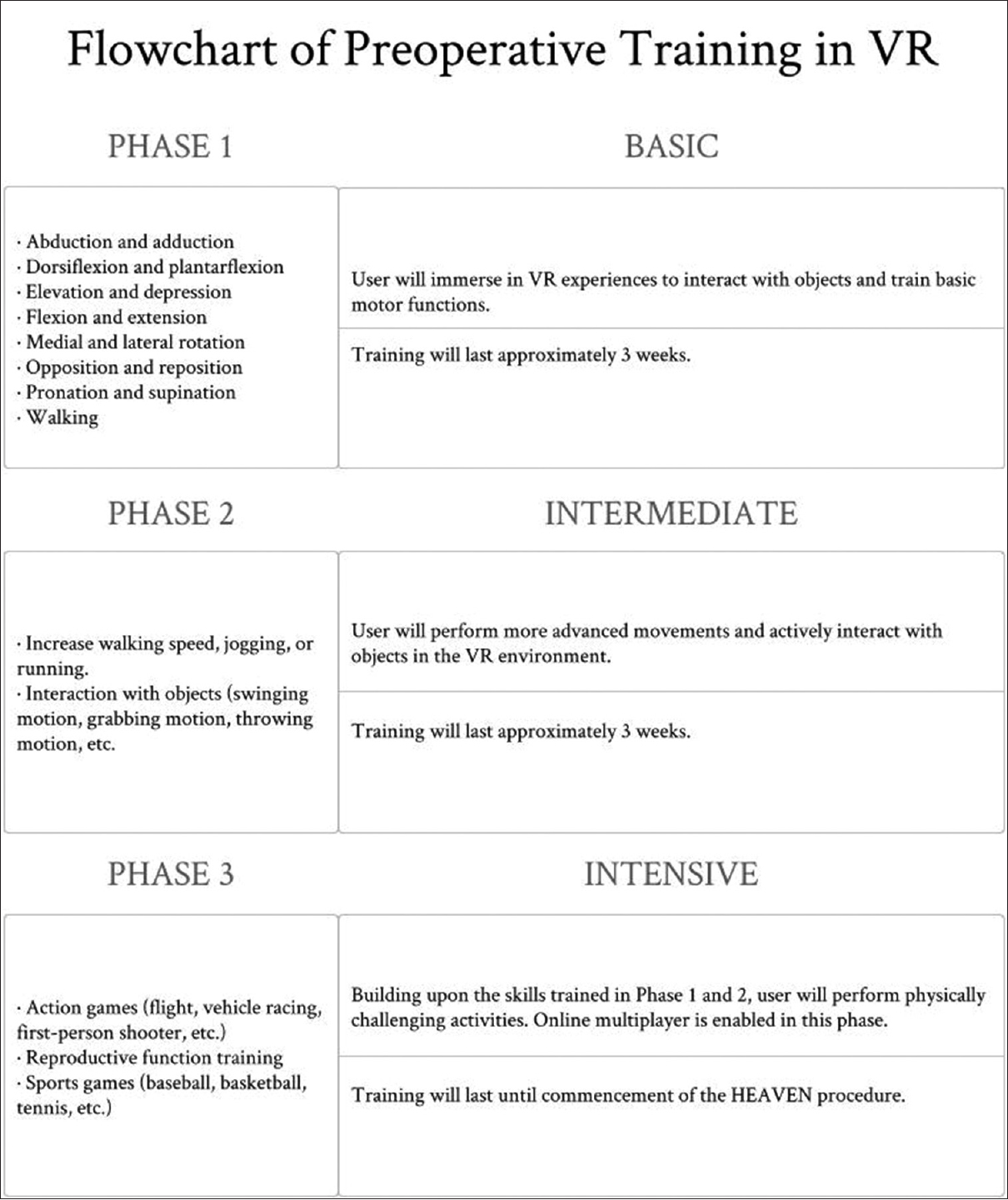
The computer equipment required for this training will be a VR capable computer, computer peripherals, VR head-mounted display, position tracking sensors, and vibration feedback controllers. The VR experiences will be a combination of commercially available games as well as the creation of customized VR experiences that will include activities that emphasize functional training. Physical intensity of activities in the VR experiences will depend on the level of injury of the user. The following phases will be used as a guideline for training various functional skills:
Phase 1: Basic
Flexion and extension Abduction and adduction Medial and lateral rotation Elevation and depression Pronation and supination Dorsiflexion and plantarflexion Opposition and reposition Walking.
Phase 2: Intermediate
Increase walking speed, jogging, or running Interaction with objects (swinging motion, grabbing motion, throwing motion, etc.).
Phase 3: Intensive
Action games (flight, vehicle racing, first-person shooter, etc.) Sports games (baseball, basketball, tennis, etc.) Reproductive function training.
Virtual reality capable computer and peripherals
It is essential to use a computer that is VR capable, i.e., the computer is capable of running VR experiences smoothly without causing a framerate lag, which can lead to disorientation and motion sickness. Guidelines for building a VR-Ready computer are widely available online.[
Virtual reality head-mounted display
Immersive VR hardware such as HTC Vive® and Oculus Rift® will be used for this proposal. Immersive VR offers the most realistic visualization of the VR environment and will be ideal for providing the user with the most lifelike experience.
Technologies for performing actions in virtual reality
There are several commercial technologies that the user can use to interact with objects and to enable navigation within a virtual environment. The following products are suggested for use in the VR training – HTC's Vive® vibration feedback controllers or Oculus’ Touch® can be used for hand movement tracking. Both of these controllers include triggers and buttons that enable interaction with the features of the VR experience. Using these controllers, the user will be able to interact with objects and interfaces in VR by performing natural hand and arm movements. The Virtuix Omni™ can be used for navigating VR using natural leg movements.[
Maintaining user engagement
VR experiences are interactive and entertaining; however, repetition of the same tasks for a long period of time can lead to disengagement.[
Progress tracking
The recipient will train in VR for 3 months before the CSA takes place. In addition, the intensity of the activities will be increased over time depending on progress results and readiness of the recipient. Progress can be tracked through improvements in game scores, surface electromyography (EMG) recordings, and observing and surveying the user. Improvements in scores will serve as an indicator of the user becoming more proficient at the tasks when scores are consistently rising. Surface EMG electrodes will be attached to the user's arms and legs for measurements on muscle electrical activity. Improvements in electrical potential generated by movements can translate to effective training. Observing the user and recording improvements such as increased time spent training and user movement appearing more natural can be indicators of effective training. The use of a survey or questionnaire regarding the user's comfort level, motivation level, and opinion on difficulty of the different experiences can also serve as a method for progress tracking.
Physical therapy gait frame
The neurologically impaired user will need assistance in maintaining a standing position while training. The IH-GAIT Frame Model provided by LL Corpus Cogere, Inc.[
Reproductive function training
The fundamental purposes of HEAVEN are the extension and propagation of life.[
To provide the recipient with a truly immersive experience of intercourse, the VR experience will be supplemented with artificial stimulation. Artificial stimulators will be used in tandem with the VR experience and provide the recipient with stimulation, which is in sync with the experience. Administering artificial stimulation will be achieved by electrostimulation. Several studies have noted the safe use of electrostimulation, via electrodes and probes, for sex rehabilitation in both male and female patients.[
PROPOSED SCENARIO FOR PREOPERATIVE VIRTUAL REALITY TRAINING
The following serves as a possible scenario to give the reader a feel for the VR training [
HEAVEN recipient will be the user of the preoperative VR training. The setup for this method is presented in Figures
Figure 2
User will be strapped into the body harness of the IH-GAIT Frame Model to maintain a standing position. The user will wear either the Vive® or Rift® head-mounted displays. This setup allows upper-body interaction with the VR environment using either the Vive® vibration feedback controllers or Touch®
Figure 3
The user will be using either the Vive® or Rift® head-mounted display while strapped into the body harness of the IH-GAIT Frame Model. This setup allows both upper and lower-body interaction with the VR environment using either the Vive® vibration feedback controllers or Oculus Touch®, and the Virtuix Omni™ locomotion simulator
CONCLUSION
Immersive VR experiences supplemented with haptic feedback provide a new approach in neurorehabilitation; thus, different combinations of VR technologies, haptic feedback technologies, and other rehabilitation assisting-tools need to be tested. Further examination is needed to determine which combination of VR and haptic feedback technologies will be optimal for preoperative training for CSA due to the lack of literature that is currently available on this subject. It will be necessary to experiment with different technologies to determine the ideal combination for providing the most realistic imitation of natural movements and stimulations. Nonetheless, the methodology proposed in this literature should be sufficient in realistically replicating the sensations associated with bodily movement and appropriately preparing the recipient of HEAVEN.
The underlying concepts in this proposed methodology are beneficial for neurorehabilitation due to VR's ability to increase motivation, improve biopsychosocial status, and encourage neuroplasticity in SCI and stroke patients. However, it is important to recognize that rehabilitation is not the main goal of this proposal. The objective is to train the recipient of HEAVEN prior to cephalic exchange for the purpose of psychological preparation. Because motor memory and neuroplasticity is consolidated primarily within the CNS,[
To conclude, the HEAVEN procedure is an endeavor that requires the readiness of not only the medical specialists, but also the recipient. It is absolutely necessary for the recipient to be prepared emotionally and psychologically. Properly preparing the recipient prior to CSA requires VR experiences accompanied with haptic feedback. The combination of these technologies will serve as protection against unexpected psychological reactions and successfully prepare the recipient for life after HEAVEN.
Financial support and sponsorship
Inventum Digital, Inc.
Conflicts of interest
There are no conflicts of interest.
Video is Available on: www.surgicalneurologyint.com
Acknowledgements
The authors of this literature wish to thank the following individuals for their support and encouragement: Sanjay Singh, PhD, James S. Walter, PhD, Raymond Dieter, Jr., MD, Sergio Canavero, MD, Xiaoping Ren, MD, Chad McCaldwell, and the Martin-Bech family. We express our appreciation to LL Corpus Cogere, Inc. for providing the IH-GAIT Frame Model. We would also like to extend our gratitude to Valery Spiridonov for his philanthropic and courageous act of volunteering to be the first recipient of HEAVEN.
References
1. Adamovich S, Fluet GG, Merians AS, Mathai A, Qiu Q. Incorporating haptic effects into three-dimensional virtual environments to train the hemiparetic upper extremity. IEEE Trans Neural Syst Rehabil Eng. 2009. 17: 512-20
2. Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002. 34: 1011-20
3. Azadzoi KM, Siroky MB. Neurologic Factors in Female Sexual Function and Dysfunction. Korean J Urol. 2010. 51: 443-9
4. Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006. 86: 1406-25
5. Benazzi F. Is overactivity the core feature of hypomania in bipolar II disorder?. Psychopathology. 2007. 40: 54-60
6. Benazzi F. What is hypomania? Tetrachoric factor analysis and kernel estimation of DSM-IV hypomanic symptoms. J Clin Psychiatry. 2009. 70: 1514-21
7. Benazzi F. Bipolar II disorder: Epidemiology, diagnosis and management. CNS Drugs. 2007. 21: 727-40
8. Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006. 51: 823-34
9. Burdea GC. Virtual rehabilitation--benefits and challenges. Methods Inf Med. 2003. 42: 519-23
10. Bruehlmeier M, Dietz V, Leenders KL, Roelcke u, Missimer J, Curt A. How does the human brain deal with a spinal cord injury?. Eur J Neurosci. 1998. 10: 3918-22
11. Canavero S. HEAVEN: The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
12. Canavero S. Sex in heaven. Surg Neurol Int. 2016. 7: 49-
13. Canavero S, Ren X. The Spark of Life: Engaging the Cortico-Truncoreticulo-Propriospinal Pathway by Electrical Stimulation. CNS Neurosci Ther. 2016. 22: 260-1
14. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
15. Castro A, Diaz F, Sumich A. Long-term neuroplasticity in spinal cord injury patients: A study on movement-related brain potentials. Int J Psychophysiol. 2013. 87: 205-14
16. Last accessed on 2016 Aug 30. Available from: http://www.cnet.com/ .
17. Colpaert L, Muller D, Fayant MP, Butera F. A mindset of competition versus cooperation moderates the impact of social comparison on self-evaluation. Front Psychol. 2015. 6: 1337-
18. Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J Physiother. 2015. 61: 117-24
19. Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ. Harnessing neuroplasticity for clinical applications. Brain. 2011. 134: 1591-609
20. Creasey GH, Craggs MD. Functional electrical stimulation for bladder, bowel, and sexual function. Handb Clin Neurol. 2012. 109: 247-57
21. Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014. 137: 654-67
22. Dimbwadyo-Terrer I, Trincado-Alonso F, de Los Reyes-Guzmán A, Aznar MA, Alcubilla C, Pérez-Nombela S. Upper limb rehabilitation after spinal cord injury: A treatment based on a data glove and an immersive virtual reality environment. Disabil Rehabil Assist Technol. 2016. 11: 462-7
23. Dimbwadyo-Terrer I, Gil-Agudo A, Segura-Fragoso A, de los Reyes-Guzmán A, Trincado-Alonso F, Piazza S. Effectiveness of the Virtual Reality System Toyra on Upper Limb Function in People with Tetraplegia: A Pilot Randomized Clinical Trial. Biomed Res Int 2016. 2016. p.
24. Donati AR, Shokur S, Morya E, Campos DS, Moioli RC, Gitti CM. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci Rep. 2016. 6: 30383-
25. Henderson AR. Psychology of hospitalized patients. J Natl Med Assoc. 1976. 68: 378-83
26. Last accessed on 2016 Aug 23. Available from: https://www.htcvive.com/us/ .
27. Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: A longitudinal fMRI study. Neurorehabil Neural Repair. 2007. 21: 527-38
28. Jones ML, Harness E, Denison P, Tefertiller C, Evans N, Larson CA. Activity-based Therapies in Spinal Cord Injury: Clinical Focus and Empirical Evidence in Three Independent Programs. Top Spinal Cord Inj Rehabil. 2012. 18: 34-42
29. Kiper P, Agostini M, Luque-Moreno C, Tonin P, Turolla A. Reinforced Feedback in Virtual Environment for Rehabilitation of Upper Extremity Dysfunction after Stroke: Preliminary Data from a Randomized Controlled Trial. Biomed Res Int 2014. 2014. p.
30. Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006. 29: 58-64
31. Levin MF, Magdalon EC, Michaelsen SM, Quevedo AA. Quality of Grasping and the Role of Haptics in a 3-D Immersive Virtual Reality Environment in Individuals With Stroke. IEEE Trans Neural Syst Rehabil Eng. 2015. 23: 1047-55
32. Last accessed on 2016 Aug 23. Available from: http://llcorpus.com/ .
33. Lohse KR, Hilderman CG, Cheung KL, Tatla S, Van der Loos HF. Virtual reality therapy for adults post-stroke: A systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS One. 2014. 9: e93318-
34. Luque-Moreno C, Ferragut-Garcías A, Rodríguez-Blanco C, Heredia-Rizo AM, Oliva-Pascual-Vaca J, Kiper P. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. Biomed Res Int 2015. 2015. p.
35. Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008. 45: 229-40
36. Merbitz NH, Westie K, Dammeyer JA, Butt L, Schneider J. After critical care: Challenges in the transition to inpatient rehabilitation. Rehabil Psychol. 2016. 61: 186-200
37. Michal M, Adler J, Wiltink J, Reiner I, Tschan R, Wölfling K. A case series of 223 patients with depersonalization-derealization syndrome. BMC Psychiatry. 2016. 16: 203-
38. Mosadeghi S, Reid MW, Martinez B, Rosen BT, Spiegel BM. Feasibility of an Immersive Virtual Reality Intervention for Hospitalized Patients: An Observational Cohort Study. JMIR Ment Health. 2016. 3: e28-
39. Nappi RE, Ferdeghini F, Abbiati I, Vercesi C, Farina C, Polatti F. Electrical stimulation (ES) in the management of sexual pain disorders. J Sex Marital Ther. 2003. 29: 103-10
40. Last accessed on 2016 Aug 23. Available from: https://www.oculus.com/ .
41. Onifer SM, Zhang O, Whitnel-Smith LK, Raza K, O’Dell CR, Lyttle TS. Horizontal Ladder Task-Specific Re-training in Adult Rats with Contusive Thoracic Spinal Cord Injury. Restor Neurol Neurosci. 2011. 29: 275-86
42. Phillips ML, Medford N, Senior C, Bullmore ET, Suckling J, Brammer MJ. Depersonalization disorder: Thinking without feeling. Psychiatry Res. 2001. 108: 145-60
43. Ren X, Canavero S. Human head transplantation: Where do we stand and a call to arms. Surg Neurol Int. 2016. 7: 11-
44. Reutens S, Nielsen O, Sachdev P. Depersonalization disorder. Curr Opin Psychiatry. 2010. 23: 278-83
45. Rodrigues-Baroni JM, Nascimento LR, Ada L, Teixeira-Salmela LF. Walking training associated with virtual reality-based training increases walking speed of individuals with chronic stroke: Systematic review with meta-analysis. Braz J Phys Ther. 2014. 18: 502-12
46. Shafik A, Shafik AA, Shafik IA, El Sibai O. Percutaneous Perineal Electrostimulation Induces Erection: Clinical Significance in Patients With Spinal Cord Injury and Erectile Dysfunction. J Spinal Cord Med. 2008. 31: 40-3
47. Sheehy L, Taillon-Hobson A, Sveistrup H, Bilodeau M, Fergusson D, Levac D. Does the addition of virtual reality training to a standard program of inpatient rehabilitation improve sitting balance ability and function after stroke? Protocol for a single-blind randomized controlled trial. BMC Neurol. 2016. 16: 42-
48. Sin H, Lee G. Additional virtual reality training using Xbox Kinect in stroke survivors with hemiplegia. Am J Phys Med Rehabil. 2013. 92: 871-80
49. Teo WP, Muthalib M, Yamin S, Hendy AM, Bramstedt K, Kotsopoulos E. Does a Combination of Virtual Reality, Neuromodulation and Neuroimaging Provide a Comprehensive Platform for Neurorehabilitation? - A Narrative Review of the Literature. Front Hum Neurosci. 2016. 10: 284-
50. Villiger M, Bohli D, Kiper D, Pyk P, Spillmann J, Meilick B. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair. 2013. 27: 675-83
51. Last accessed on 2016 Aug 23. Available from: http://www.virtuix.com/ .
52. Wallen K, Lloyd EA. Female Sexual Arousal: Genital Anatomy and Orgasm in Intercourse. Horm Behav. 2011. 59: 780-92
53. Yurong M, Chen P, Li L, Huang D. Virtual reality training improves balance function. Neural Regen Res. 2014. 9: 1628-34
54. Zimmerli L, Jacky M, Lünenburger L, Riener R, Bolliger M. Increasing patient engagement during virtual reality-based motor rehabilitation. Arch Phys Med Rehabil. 2013. 94: 1737-46