- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
- Department of Neuro-Oncology, MD Anderson Cancer Center, Houston, Texas, United States.
- Department of Neurosurgery, University of Florida, Gainesville, Florida, United States.
Correspondence Address:
Ganesh Rao
Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_174_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: A. Basit Khan1, Carlos Kamiya Matsuoka2, Sungho Lee1, Maryam Rahman3, Ganesh Rao1. Prolonged survival after laser interstitial thermal therapy in glioblastoma. 17-May-2021;12:228
How to cite this URL: A. Basit Khan1, Carlos Kamiya Matsuoka2, Sungho Lee1, Maryam Rahman3, Ganesh Rao1. Prolonged survival after laser interstitial thermal therapy in glioblastoma. 17-May-2021;12:228. Available from: https://surgicalneurologyint.com/surgicalint-articles/10811/
Abstract
Background: Glioblastoma (GBM) is the most common primary malignant brain tumor in adults. Management includes surgical resection followed by chemoradiation, and prognosis remains poor. Surgical resection is not possible for some deep-seated or eloquent tumors. Laser interstitial thermal therapy (LITT) has emerged as a new, minimally invasive surgical option for deep-seated GBM.
Case Description: We report a case of newly diagnosed thalamic GBM managed with LITT followed by radiation and chemotherapy.
Conclusion: The patient remains well at 50-month post-LITT, indicating a potentially unique durability of LITT treatment in GBM.
Keywords: Glioblastoma, Laser interstitial thermal therapy, Survival
INTRODUCTION
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults with an age-adjusted annual incidence of 3.22/100,000 population.[
Laser interstitial thermal therapy (LITT) is a technique used to thermally ablate tumors in a minimally invasive fashion. The use of laser-mediated interstitial thermal ablation was first demonstrated in 1983, and the clinical utility of this technology was improved by the development of magnetic resonance (MR) thermography.[
CLINICAL CASE
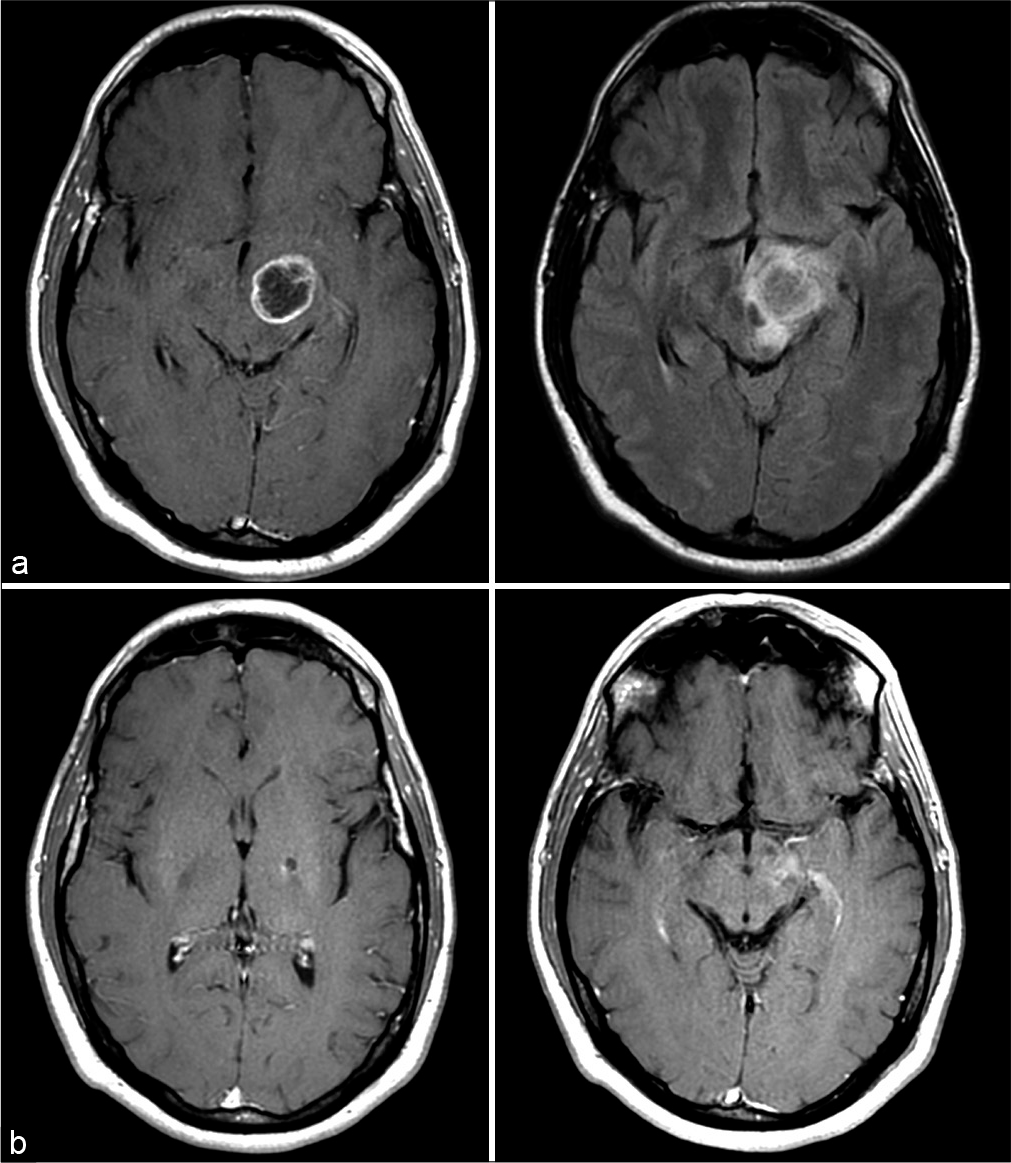
A previously healthy 32-year-old man presented to our institution for a second opinion after undergoing biopsy of a left thalamic lesion which revealed GBM. He initially presented to an outside hospital with a complaint of progressive clumsiness of the right hand and foot. MRI of the brain revealed a solitary ring-enhancing lesion with associated increased FLAIR signal in the left thalamopeduncular region [
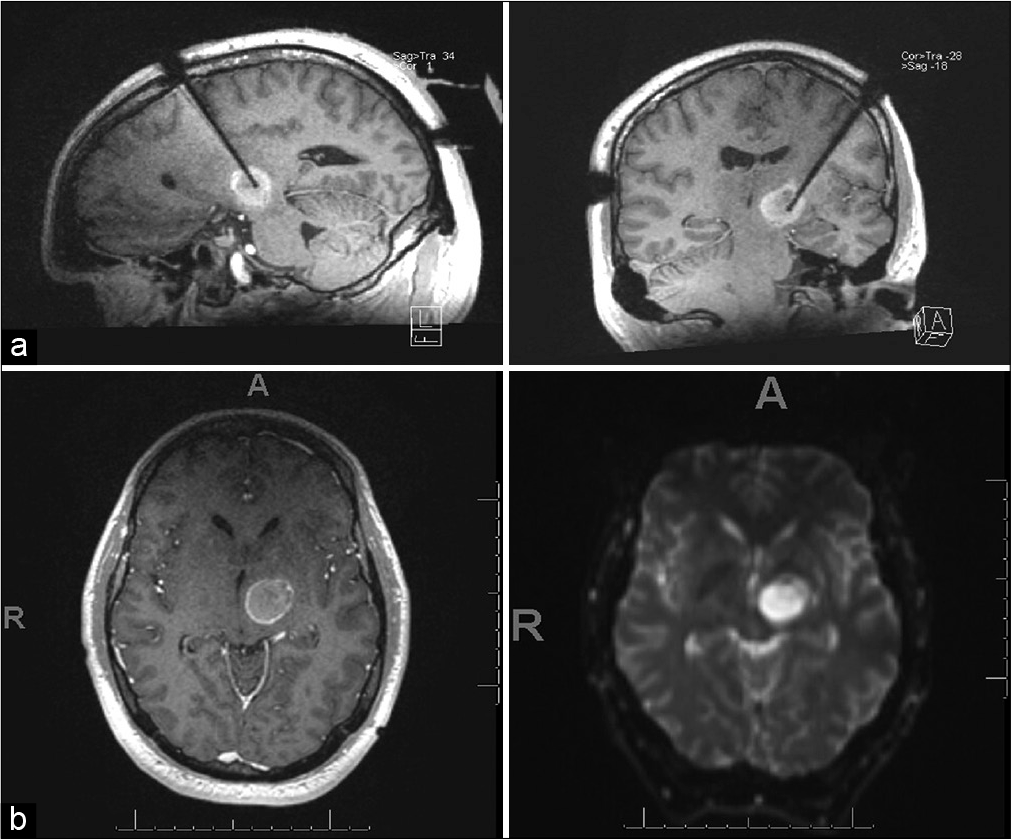
The LITT procedure was performed in our intraoperative MRI suite using image-guided stereotactic lead placement using the ClearPoint system (Clearpoint Neuro). For the ablation, we utilized the NeuroBlate system (Monteris). The laser ablation was carried out under real-time MR thermography [
Postoperatively, the patient received intensity-modulated radiation therapy to the resection cavity with a total dose of 60 Gy in 30 fractions starting on postoperative day 13. He did not receive concurrent chemotherapy. Thereafter, he was given adjuvant temozolomide at 200 mg/m2/day, days 1–5 every 28-day cycle, for 12 cycles. Most recently, his MRI from 47.5 months after initial biopsy and 46.9 months after LITT showed only a small cavitation with minimal enhancement and FLAIR signal, likely as a result of LITT [
DISCUSSION
Observations
Here, we report a case of prolonged survival of a patient with thalamic GBM treated with LITT and subsequent adjuvant treatment that concluded 1 year after diagnosis. The patient remains disease free over 50 months from diagnosis. While an outlier, this case represents the potential durable treatment effect of LITT in newly diagnosed GBM.
Lessons
The use of LITT for treatment of GBM, whether primary or recurrent, is an emerging surgical option for tumor control. When considering deep-seated tumors, a reasonable comparison is to patients who were previously allocated to biopsy-only options. Patients undergoing concurrent chemoradiation after biopsy only had a median overall survival (OS) of 9.4 months versus 15.8 months in patients who underwent tumor resection.[
Beyond direct tumor control, alterations in GBM pathobiology in response to LITT are actively being studied. One of the challenges to treating GBM is delivering chemotherapy across the blood–brain barrier. Recent studies have shown that LITT treatment can increase permeability of the blood–brain barrier in a time-dependent fashion.[
The genetic landscape of GBM is diverse with certain genetic alterations portending variably good or bad prognoses. Per the WHO 2016, GBM diagnoses are segregated on the basis of IDH mutational status.[
CONCLUSION
In summary, we report a unique case of prolonged survival and excellent radiographic response using LITT to treat a thalamic GBM. GBM in deep-seated locations remains challenging neuro-oncological problems as resection is often not possible. The published data regarding LITT are sparse with mixed result. This case highlights the need for higher quality research regarding LITT in GBM. Moreover, this report suggests that LITT treatment in GBM may offer durable treatment response beyond the current standard of care.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
None.
References
1. Bown SG. Phototherapy in tumors. World J Surg. 1983. 7: 700-9
2. de Poorter J, de Wagter C, de Deene Y, Thomsen C, Ståhlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: In vivo results in human muscle. Magn Reson Med. 1995. 33: 74-81
3. Ivan ME, Mohammadi AM, de Deugd N, Reyes J, Rodriguez G, Shah A. Laser ablation of newly diagnosed malignant gliomas: A meta-analysis. Neurosurgery. 2016. 79: S17-23
4. Kamath AA, Friedman DD, Akbari SH, Kim AH, Tao Y, Luo J. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: Safety, efficacy, and outcomes. Neurosurgery. 2019. 84: 836-43
5. Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016. 11: e0148613
6. Li QJ, Cai JQ, Liu CY. Evolving molecular genetics of glioblastoma. Chin Med J (Engl). 2016. 129: 464-71
7. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
8. Man J, Shoemake JD, Ma T, Rizzo AE, Godley AR, Wu Q. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015. 75: 1760-9
9. Mensel B, Weigel C, Hosten N. Laser-induced thermotherapy. Recent Results Cancer Res. 2006. 167: 69-75
10. Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, UroCoste E, Amiel-Benouaich A. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017. 19: 1127-34
11. Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015. 38: E13
12. Mohammadi AM, Sharma M, Beaumont TL, Juarez KO, Kemeny H, Dechant C. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: A multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019. 85: 762-72
13. Montemurro N, Anania Y, Cagnazzo F, Perrini P. Survival outcomes in patients with recurrent glioblastoma treated with laser interstitial thermal therapy (LITT): A systematic review. Clin Neurol Neurosurg. 2020. 195: 105942
14. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-100
15. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
16. Stafford RJ, Fuentes D, Elliott AA, Weinberg JS, Ahrar K. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010. 38: 79-100
17. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
18. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016. 41: E12
19. Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W. MGMT promoter methylation in malignant gliomas: Ready for personalized medicine?. Nat Rev Neurol. 2010. 6: 39-51
20. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009. 360: 765-73