- Department of Neurology, Yale School of Medicine, New Haven, Connecticut, United States.
- Department of Radiology and Biomedical Imaging, Yale University, New Haven, Connecticut, United States.
Correspondence Address:
Adeel Shakil Zubair, Department of Neurology, Yale School of Medicine, New Haven, Connecticut, United States.
DOI:10.25259/SNI_499_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Adeel Shakil Zubair1, Michele Johnson2, Emily Jean Gilmore1. Pseudosubarachnoid hemorrhage. 29-Jan-2022;13:32
How to cite this URL: Adeel Shakil Zubair1, Michele Johnson2, Emily Jean Gilmore1. Pseudosubarachnoid hemorrhage. 29-Jan-2022;13:32. Available from: https://surgicalneurologyint.com/surgicalint-articles/11364/
Abstract
Background: Imaging findings postcardiac arrest can influence medical decisions and have impact on discussions with caregivers.
Case Description: A 44-year-old female presented to the emergency department and later had an in hospital cardiac arrest. Return of spontaneous circulation was obtained and the patient was taken for imaging which was read as diffuse bilateral subarachnoid hemorrhage. Magnetic resonance imaging of the brain performed later showed no evidence of subarachnoid hemorrhage nor was it seen later on autopsy.
Conclusion: Pseudosubarachnoid hemorrhage can be seen in patients with diffuse cerebral edema, often in the setting of either hypercarbia or severe acute diffuse injury.
Keywords: Neurocritical care, Pseudosubarachnoid hemorrhage, Subarachnoid hemorrhage
A 44-year-old woman with bipolar disorder presented to the emergency department (ED) in the midst of a manic episode. She was anxious, tangential, hyperverbal, and delusional prompting transfer to psychiatry’s ED crisis unit to await admission. Hours later, during a routine check, she was found unresponsive and pulseless. Cardiac monitoring revealed pulseless electrical activity; cardiopulmonary resuscitation was initiated with return of spontaneous circulation (ROSC) after 16 min. Computed tomography (CT) brain, performed immediately after ROSC, was reported as diffuse bilateral subarachnoid hemorrhage [
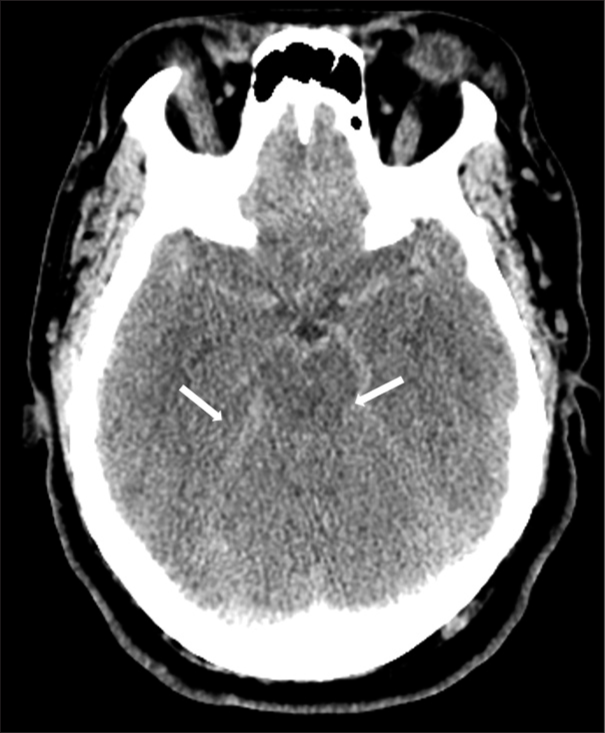
Figure 1:
Careful review of the computed tomography images reveals hyperdense appearance of the Sylvian and perimesencephalic vasculature as well as the tentorial leaflets. Note that the suprasellar cistern is not filled with hyperdensity nor is there hyperdensity in the sulci or layering of blood over the tentorium as would be evident with subarachnoid hemorrhage. Most importantly, one should note the loss of gray/white matter differentiation in this 44-year-old patient. The midbrain is low in density, the cisternal spaces are reduced in size, and the cerebellum demonstrates no gray/white matter differentiation. The relatively hyperdense appearance of vessels within the Sylvian and perimesencephalic cisterns, the reduced size of the basal cisterns, and the parenchymal hypodensity are indicative of diffuse anoxic injury – not subarachnoid hemorrhage.
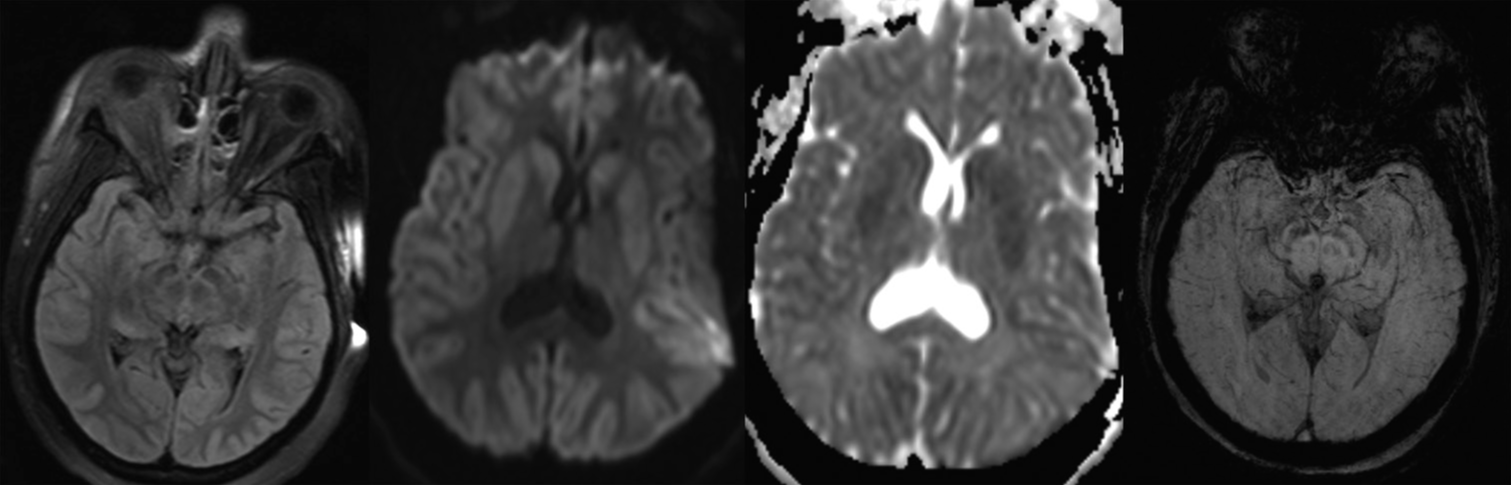
Figure 2:
Magnetic resonance imaging – hyperacute protocol (susceptibility-weighted imaging, fluid-attenuation inversion recovery [FLAIR], diffusion-weighted imaging [DWI], and apparent diffusion coefficient [ADC] sequence). (1) FLAIR images demonstrate no evidence of abnormal signal within the subarachnoid fluid spaces and no flow-related enhancement. (2 and 3) Diffuse supratentorial restricted diffusion is noted with involvement of the deep nuclei (caudate, lentiform, and pulvinar) on DWI and ADC sequences. (4) Susceptibility-weighted imaging image demonstrates no blooming artifact or low signal intensity within the basal cisterns or sulci as would be identified with subarachnoid hemorrhage.
PANEL A: COMPUTED TOMOGRAPHY IMAGING OF THE HEAD
Careful review of the CT images reveals hyperdense appearance of the Sylvian and perimesencephalic vasculature as well as the tentorial leaflets. Note that the suprasellar cistern is not filled with hyperdensity nor is there hyperdensity in the sulci or layering of blood over the tentorium as would be evident with subarachnoid hemorrhage. Most importantly, one should note the loss of gray/white matter differentiation in this 44-year-old patient. The midbrain is low in density, the cisternal spaces are reduced in size, and the cerebellum demonstrates no gray/white matter differentiation. The relatively hyperdense appearance of vessels within the Sylvian and perimesencephalic cisterns, the reduced size of the basal cisterns, and the parenchymal hypodensity are indicative of diffuse anoxic injury – not subarachnoid hemorrhage.
PANEL B: MAGNETIC RESONANCE IMAGING – HYPERACUTE PROTOCOL (SUSCEPTIBILITY-WEIGHTED IMAGING, FLUID-ATTENUATION INVERSION RECOVERY (FLAIR), DIFFUSION-WEIGHTED IMAGING (DWI), AND APPARENT DIFFUSION COEFFICIENT (ADC) SEQUENCE)
FLAIR images demonstrate no evidence of abnormal signal within the subarachnoid fluid spaces and no flow-related enhancement Diffuse supratentorial restricted diffusion is noted with involvement of the deep nuclei (caudate, lentiform, and pulvinar) on DWI and ADC sequences SWI image demonstrates no blooming artifact or low signal intensity within the basal cisterns or sulci as would be identified with subarachnoid hemorrhage.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agha A, Al-Hakami M. A case report of pseudo-subarachnoid hemorrhage. Maedica (Bucur). 2011. 6: 210-2
2. Given CA, Burdette JH, Elster AD, Williams DW. Pseudo-subarachnoid hemorrhage: A potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003. 24: 254-6
3. Joyce RR, McGee WT. Hypercapnic cerebral edema presenting in a woman with asthma: A case report. J Med Case Rep. 2011. 5: 192
4. Yuzawa H, Higano S, Mugikura S, Umetsu A, Murata T, Nakagawa A. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: Characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol. 2008. 29: 1544-9