- Department of Surgery, Neurosurgery Section, University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico, United States,
- Department of Neurosurgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, United States,
- Department of Neurosurgery, Allegheny General Hospital, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Mark C. Preul, Department of Neurosurgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, United States.
DOI:10.25259/SNI_240_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giancarlo Mignucci-Jiménez1,2, Alejandro J. Matos-Cruz1,3, Irakliy Abramov2, Sahin Hanalioglu2, Melissa S. Kovacs2, Mark C. Preul2, Caleb E. Feliciano-Valls1. Puerto Rico Recurrence Scale: Predicting chronic subdural hematoma recurrence risk after initial surgical drainage. 03-Jun-2022;13:230
How to cite this URL: Giancarlo Mignucci-Jiménez1,2, Alejandro J. Matos-Cruz1,3, Irakliy Abramov2, Sahin Hanalioglu2, Melissa S. Kovacs2, Mark C. Preul2, Caleb E. Feliciano-Valls1. Puerto Rico Recurrence Scale: Predicting chronic subdural hematoma recurrence risk after initial surgical drainage. 03-Jun-2022;13:230. Available from: https://surgicalneurologyint.com/surgicalint-articles/11639/
Abstract
Background: Chronic subdural hematoma (CSDH) commonly affects older individuals and is associated with a relatively high rate of recurrence after surgery. Many studies have created grading systems to identify patients at high risk of CSDH recurrence after the initial surgery. However, no system has been adopted widely. The authors present the first CSDH grading system created from a population-based single-center data set.
Methods: A single-center Puerto Rican population-based retrospective analysis was performed on consecutive patients treated for a CSDH at a designated institution from July 1, 2017 to December 31, 2019. Univariate and multivariate analyses were used to create a CSDH recurrence grading scale. Retrospective validation was conducted on this sample population.
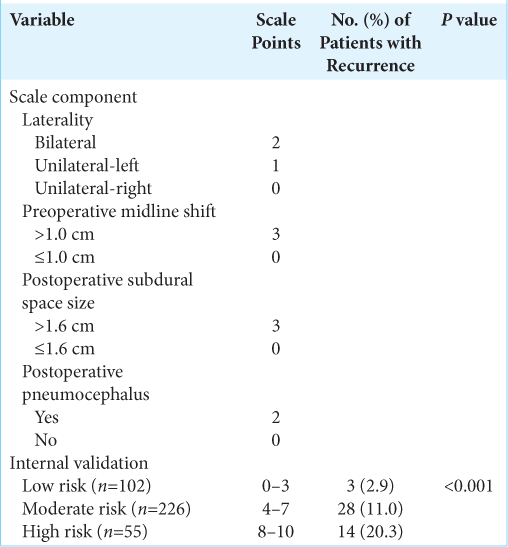
Results: The study included 428 patients. Preoperative midline shift, postoperative midline shift, and size of postoperative subdural space differed between the recurrence and nonrecurrence groups (P = 0.03, 0.002, and 0.002, respectively). A multivariate analysis was used to create a 10-point grading scale comprising four independent variables. Recurrence rates progressively increased from the low-risk (0–3 points) to high-risk (8–10 points) groups (2.9% vs. 20.3%; P
Conclusion: A 10-point grading scale for CSDH recurrence was developed with four components: preoperative midline shift (≤1 and >1 cm), laterality (bilateral, unilateral-right, and unilateral-left), size of postoperative subdural space (≤1.6 and >1.6 cm), and pneumocephalus (present or absent). Patients who scored higher on the scale had a higher risk of recurrence. This CSDH grading scale has implications for Puerto Rico and the general population as the elderly population increases worldwide.
Keywords: Chronic subdural hematoma, Grading scale, Puerto Rico, Recurrence, Subdural space
INTRODUCTION
The topic of chronic subdural hematoma (CSDH) and its recurrence has recently gained much attention. CSDH is one of the most common conditions neurosurgeons treat, estimated to occur in 3.4–5 individuals/100,000.[
CSDH is strongly associated with neurotrauma, specifically minor head trauma. Head trauma is the most important risk factor for the development of CSDH, with 50–80% of patients in large studies having a history of head trauma before presentation.[
Studies have identified demographic, comorbid, preoperative, and postoperative factors associated with CSDH recurrence.[
MATERIALS AND METHODS
Study design
The study was approved by the University of Puerto Rico Medical Sciences Campus Institutional Review Board (A0230120). Due to the retrospective design of the study and the lack of patient identifiable information, patient consent was not obtained. Electronic medical records of patients who underwent operations at the Puerto Rico Medical Center between July 1, 2017, and December 31, 2019, were retrospectively analyzed. The study inclusion criteria were as follows: age 18 years or older, CSDH diagnosed on computed tomography (CT), surgical management of CSDH, and preoperative and postoperative imaging performed at our institution. Exclusion criteria were missing preoperative or postoperative CT imaging and CT images with motion artifacts. A total of 833 patient records with the diagnosis of CSDH were reviewed; 428 patients met the study criteria and were included in the analysis. Both unilateral and bilateral hematoma cases were included in the analysis. Detailed information on medications used in the preoperative or postoperative management of CSDH (i.e., dexamethasone, atorvastatin, or tranexamic acid) was not included in the analysis.
All patients underwent surgical evacuation through a burr hole or craniotomy. If a patient required both burr hole and craniotomy drainage, the patient was assigned to the craniotomy group. If a patient presented with bilateral hematomas (n = 177) but underwent a unilateral operation, they were not included in the bilateral procedure group (61 patients who presented with bilateral hematomas were not included in the bilateral procedure group [n = 116]). Saline irrigation was used in all patients. Postoperative drainage was used at the discretion of the attending neurosurgeon.
All imaging data were obtained using the hospital’s picture archive and communication system (Carestream Vue Motion Software; Carestream, Inc., Rochester, NY). Postoperative imaging was performed on postoperative day 1. All imaging was performed with a standard CT machine and no images were obtained using a portable CT scanner. Patients who had images with motion artifacts were excluded from the study. Patients were added to the recurrence group if they fulfilled all of the following conditions: (1) reaccumulation of blood within the postoperative hematoma cavity on follow-up CT, (2) reappearance of neurological symptoms, (3) diagnostic imaging conducted at our institution, and (4) another surgical intervention at our institution. Patients who had CSDH in the postoperative space on repeat imaging but did not become symptomatic or require surgery were not considered to have had a recurrent CSDH.
Radiographic measurements
Quantitative measurements were obtained for all preoperative and postoperative images using the hospital’s picture archive and communication system. All measurements (in centimeters) were taken by a single neurosurgeon and verified by a neuroradiologist, both of whom were masked as to whether a patient belonged to the recurrence or nonrecurrence group.
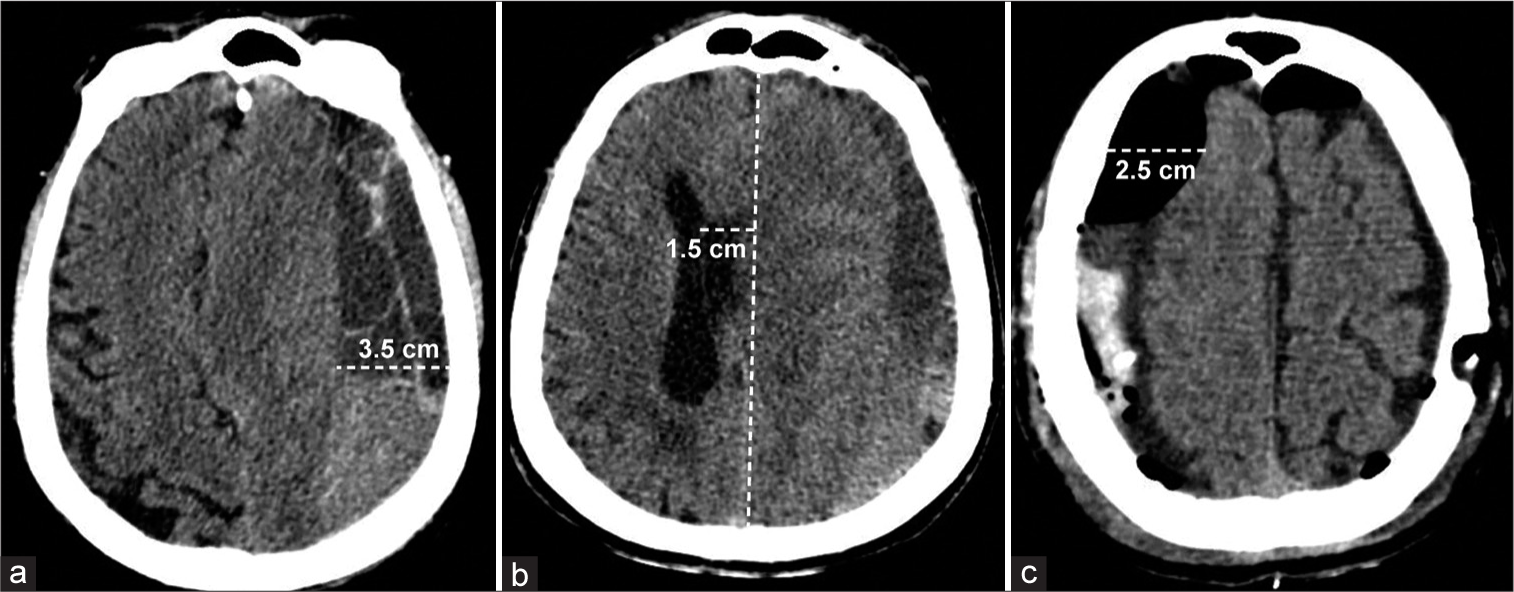
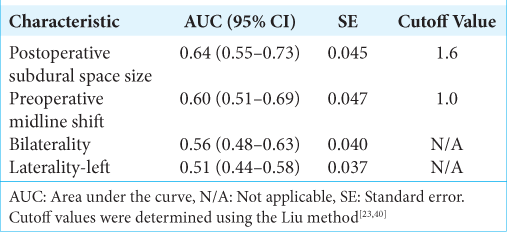
Preoperative and postoperative midline shifts were determined by drawing a line from the anterior and posterior attachments of the falx cerebri and measuring the maximal perpendicular distance of the septum pellucidum from this line, usually at the level of the foramen of Monroe, if identifiable. The size of the CSDH was defined as the maximal thickness of hematoma from the inner table of the skull to the surface of the brain in the axial view. If a patient had bilateral hematomas, the size of the CSDH refers to the larger of the two hematomas. The size of the postoperative subdural space was defined as the maximal thickness of subdural space from the inner table of the skull to the surface of the brain in the axial view. For patients who underwent a bilateral procedure, the postoperative subdural space refers to the larger of the two subdural spaces. For sizes of CSDH and postoperative subdural space, measurements were not acquired in the cranial convexity in order to prevent overestimation of distance. Examples of CSDH size, preoperative midline shift, and size of the postoperative subdural space are shown in
Figure 1:
Examples of quantitative variables in the study cohort were measured on axial CT images. (a) Measurement of the size of chronic subdural hematoma (CSDH) in a patient presenting with unilateral-left CSDH. (b) Measurement of the preoperative midline shift in a patient presenting with unilateral-left CSDH. (c) Measurement of the size of postoperative subdural space in a patient presenting with bilateral CSDH who also underwent a bilateral procedure. The white dashed line indicates the measured distance. Used with permission from Centro Médico de Puerto Rico, Administración de Servicios Médicos de Puerto Rico, San Juan, Puerto Rico.
Statistical analysis and grading-scale creation
Stata/MP 15.1 software (StataCorp LP, College Station, TX, USA) was used for the statistical analyses. Most variables exhibited skew or kurtosis, necessitating the reporting of medians instead of means in the descriptive statistics. The Chi-square test was used to assess the differences in categorical variables between patients whose CSDH recurred (recurrence) and patients whose CSDH did not recur (nonrecurrence). For the continuous variables, Mann–Whitney U tests or t-tests were used to evaluate the differences between medians and means, respectively.
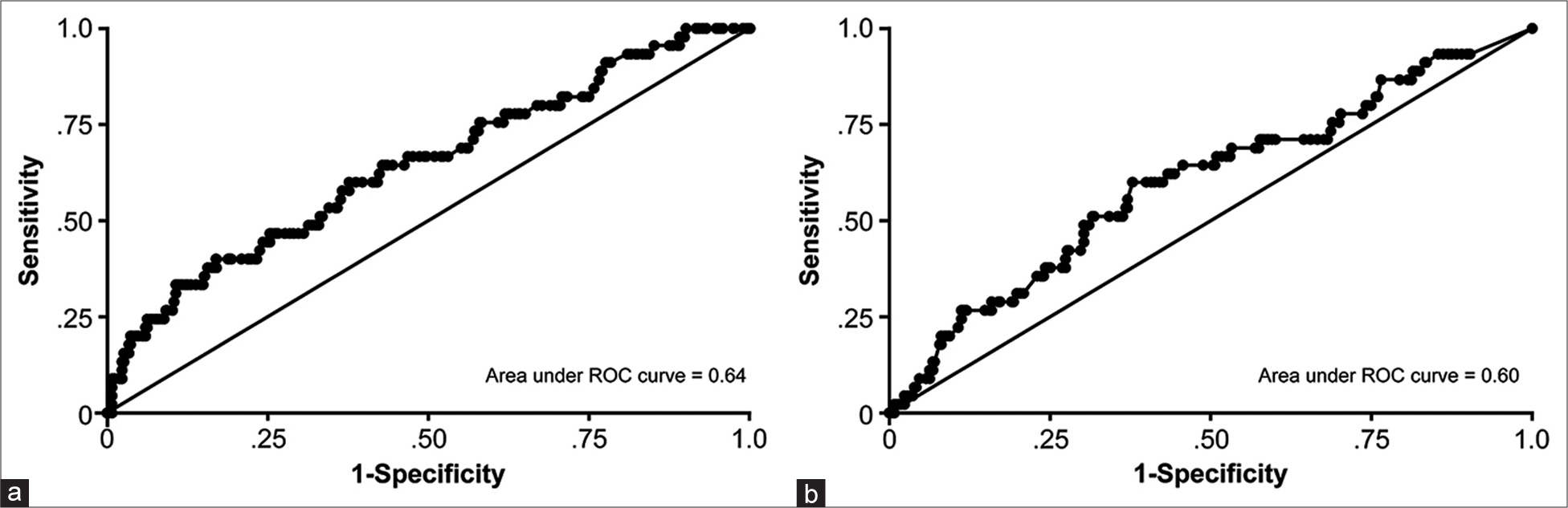
Variables with P < 0.20 in univariate analyses were tested for inclusion in a multivariate logistic regression model. The logistic regression model provided odds ratios (ORs) with 95% confidence intervals and β regression coefficients. A receiver operating characteristic (ROC) curve was created from the multivariate logistic regression model. The area under the curve (AUC) and corresponding 95% confidence intervals were calculated. The continuous variables’ cutoff point values were calculated using CUTPT.[
RESULTS
Patient characteristics
We identified 428 patients with CSDH who met the inclusion criteria.
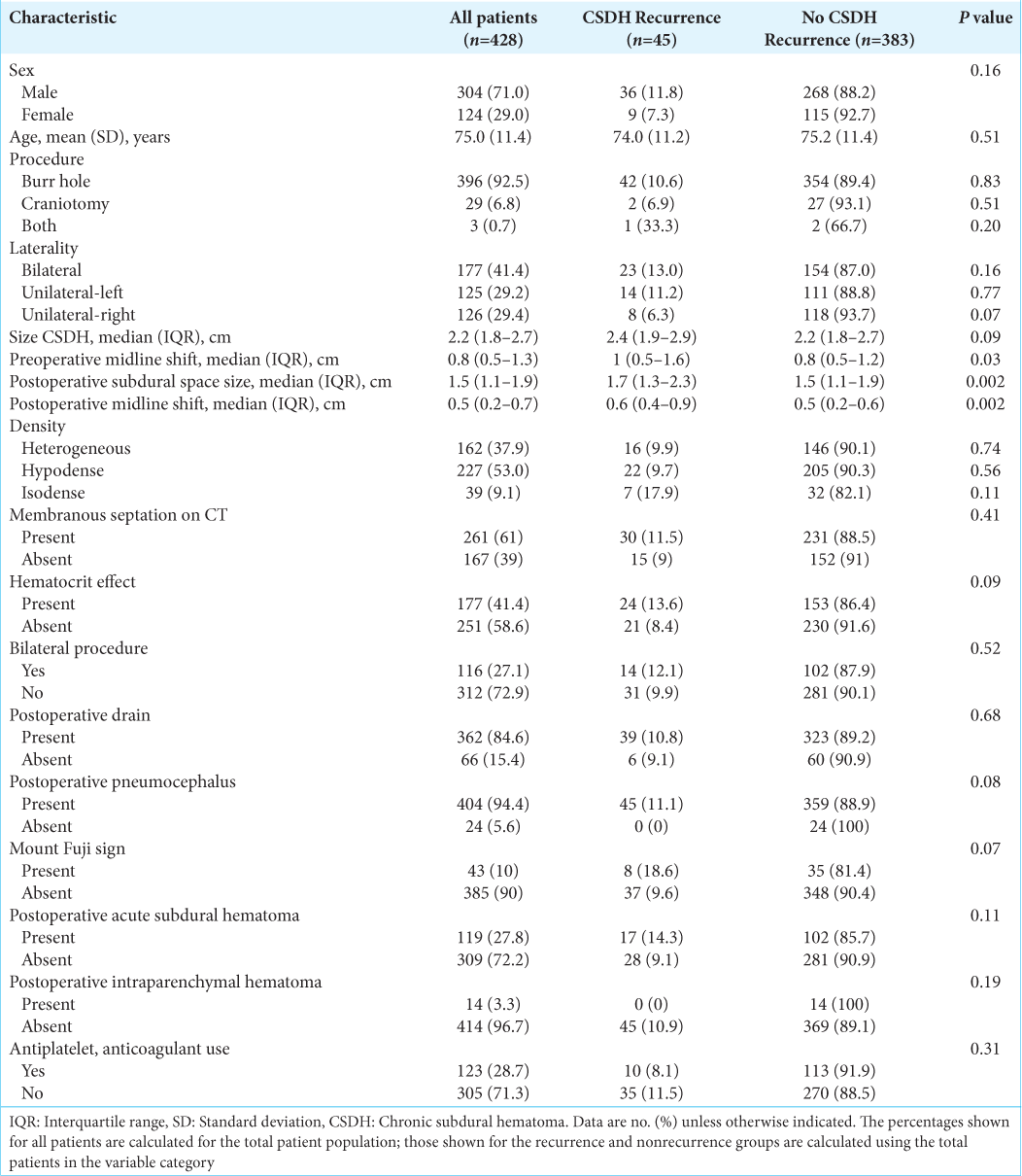
Univariate analysis
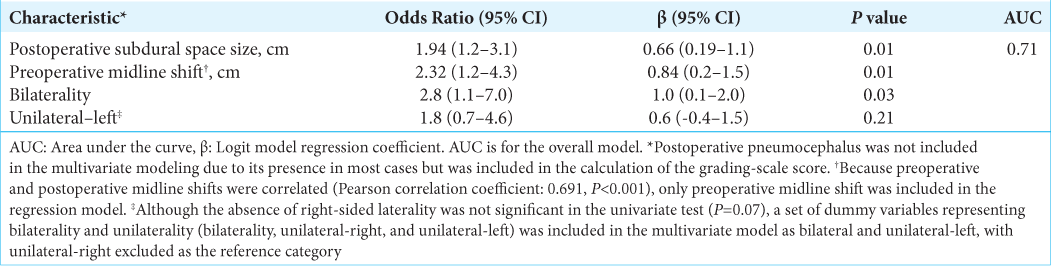
Multiple logistic regression analysis
Multivariate logistic regression results are shown in
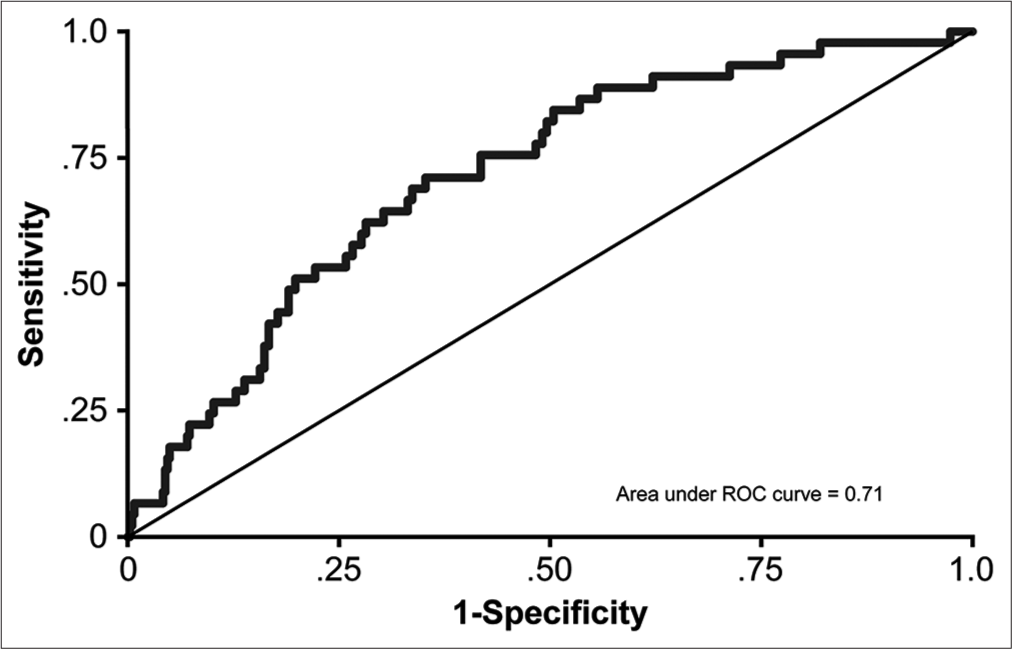
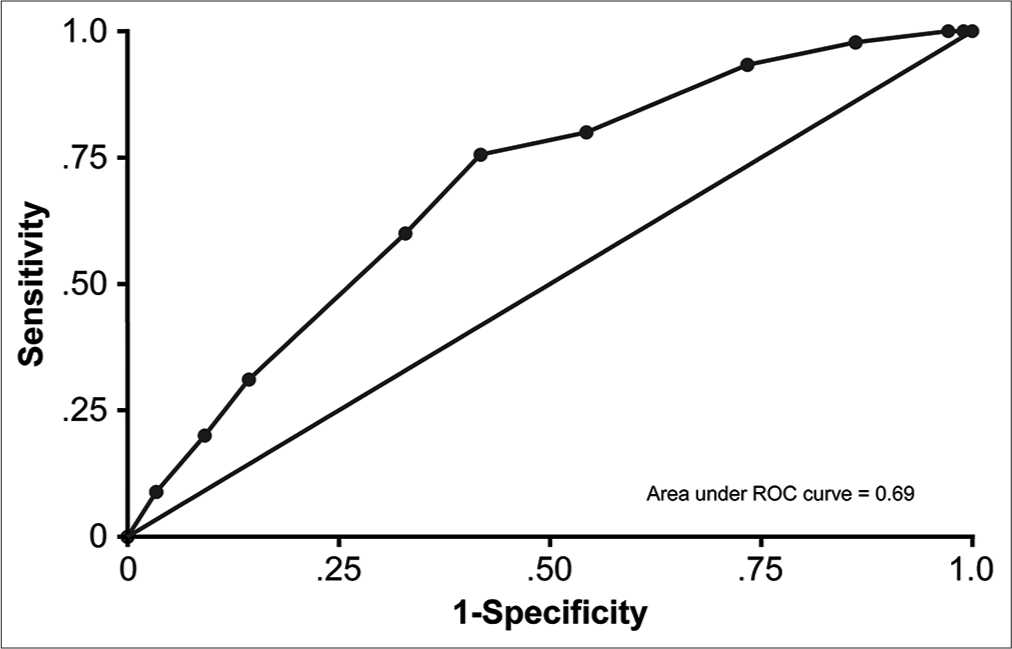
The overall AUC for the model was 0.71, categorizing it as a fair predictor.[
Internal validation and model use
DISCUSSION
This report is the first of a predictive CSDH recurrence grading scale developed on the basis of a single population data set, to the best of our knowledge. In addition, this study presents the largest sample size used to develop any CSDH recurrence prediction system (see
The derived scale may have utility in managing patients undergoing surgical evacuation of CSDH. Patients with higher scores could be at greater risk of recurrence. Our study findings may be extrapolated to the treatment centers that care for an older population. This grading system could be used to standardize patient care, improve enrollment criteria for clinical trials, determine whether a patient would benefit from adjuvant treatment (i.e., middle meningeal artery embolization), and provide reliable criteria for assessing new treatment strategies.
Factors associated with recurrence and subdural space
The univariate analysis showed that the preoperative midline shift, postoperative subdural space size, and postoperative midline shift differed significantly between the two study groups. Other studies have reported similar results.[
Increased postoperative subdural space size contributes to CSDH recurrence for many reasons. First, it allows blood to reaccumulate, and, in some cases (i.e., subdural pneumocephalus), it provides a low-pressure environment. Second, it suspends the brain and puts bridging veins at a higher risk of tearing. Finally, it prevents the adhesion between the inner and outer neomembranes created by the CSDH where adhesion of the inner and outer neomembranes is believed to be necessary for CSDH cure.[
A persistent postoperative subdural space reflects poor brain expansion that occurs with advanced age and atrophy. As patients get older, their brains lose elasticity and cannot adequately expand after being compressed. Brain atrophy is an independent factor that contributes to CSDH recurrence.[
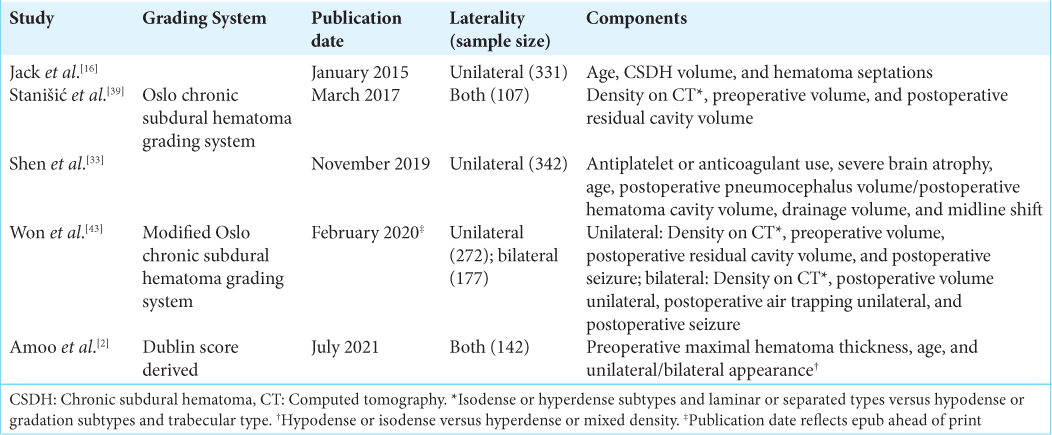
Previous grading scales for CSDH recurrence
Five CSDH recurrence or risk grading systems have been proposed.[
Our study devised a grading system similar to others that use laterality (i.e., bilateral vs. unilateral), preoperative midline shift, and postoperative pneumocephalus (i.e., postoperative air trapping). However, our use of postoperative subdural space size is unique. Our scale differs from other scales. It does not incorporate age, use of antiplatelet or anticoagulant agents, presence or absence of septations, hematoma appearance on CT (i.e., hematoma density), severe brain atrophy, postoperative seizures, drainage volume, the volume of the postoperative cavity, postoperative hematoma density, or fraction of postoperative pneumocephalus volume to postoperative hematoma cavity volume.
Our grading system comprises variables that were significantly and nonsignificantly different between the study groups — not all significant variables were included in the model. In addition, the model does not include some study variables that were used as components in the previous grading systems (e.g., age, anticoagulant or antiplatelet use, visible membranous septations, or hematoma density). We do not include them for two reasons. First, significant variables that were highly correlated with one another (e.g., preoperative and postoperative midline shift) cannot both be included in the study. The fitting of a multivariate model requires that the variables be independent. Thus, only the best-fit variable was used. Second, using multivariate logistic regression, the best combination of variables that explains the variance between our study groups was used to create our grading scale. In other words, our combination of four independent variables best explained the variation between recurrence and nonrecurrence of CSDH. The study variables used in the previous grading systems were evaluated and did not produce a significant model in our study population.
Benefits of the Puerto Rico Recurrence Scale
The unique components of our grading scale are the specification of the right and left in unilaterality and the size of the postoperative subdural space (i.e., the maximal thickness of subdural space from the inner table of the skull to the surface of the brain in the axial view). Although other scales incorporate laterality as a component, none consider the side of a unilateral hematoma. In our study, the left-sided hematoma recurrence was more prevalent than right-sided hematoma recurrence, which was used as a component of our scale (unilateral-left = 1 point). A possible explanation for this prevalence is that the surgeons treated the left side with more caution during surgery because the left hemisphere of the brain is dominant in most patients. Greater caution by the surgeon could result in inadequate drainage and a larger residual space.
Earlier studies have emphasized consideration of the subdural space (i.e., postoperative or hematoma cavity)[
Our proposed grading system is potentially superior to other scales for several reasons. First, our scale was developed using a large and single population-based data set. Therefore, the results are generalizable to other populations. Second, the study was conducted at a single center, thereby eliminating potential intercenter differences in the data. Third, our system is applicable to both unilateral and bilateral CSDH cases. Fourth, we showed that this scale has clinical value when applied retrospectively to a large sample population. Our grading system produced an AUC of 71%, which exactly mimics the AUC of the scoring system devised by Amoo et al.[
Limitations
Some limitations apply to the study. The operations were performed by five different surgeons, and the results could vary between surgeons. Our definition of CSDH recurrence is not common to all CSDH studies. Thus, some patients with radiographic evidence of recurrence who did not undergo surgery were excluded from the study. As a result, there is a potential for selection bias. Our measurement of certain variables (e.g., size of the CSDH and midline shift) might differ from those used in other studies and thus lead to differences when comparing studies. The study is retrospective and long-term follow-up data are lacking.
In addition, there are important limitations that affect some parameters used in this study. First, abuse of alcohol was cited earlier as a possible risk for recurrence. The Puerto Rican population is known to have a high rate of alcohol abuse disorder (AUD) according to the DSM-V, especially among older men (36% of men over the age of 50 have AUD).[
The use of a postoperative drain affects the volume of the subdural space. Some neurosurgeons prefer not to use a drain. Therefore, this study might not characterize the amount of subdural space and risk of recurrence due to neurosurgeon technique preferences.
Data regarding patient-specific coagulopathy were not available for many patients in this study. Thus, its association with CSDH could not be determined.
Although the presence of pneumocephalus correlated with CSDH recurrence in this study, the volume of pneumocephalus was not measured and analyzed. It makes intuitive sense that a larger amount of intracranial air, hence subdural space, could increase the risk of CSDH recurrence. These parameters are limited due to the retrospective nature of the study and a focus on other radiographic findings.
Specific medications used in the preoperative or postoperative management of patients (e.g., dexamethasone, atorvastatin, and tranexamic acid) were not considered in the present study. In many cases, detailed records of medications were unavailable.
CONCLUSION
CSDH is one of the most common neurosurgical pathologies and the rate of recurrence is high despite advances in the practice of neurosurgery. At present, there is no widely adopted grading system to predict CSDH recurrence. Our study proposes a novel scoring system (i.e., the Puerto Rico Recurrence Scale) developed through a single population-based sample at a single institution devoted to neurosurgical management of such patients. The scale has four components: laterality, preoperative midline shift, postoperative subdural space size, and postoperative pneumocephalus. The unique components of the proposed scale comprise the side of the brain in unilateral CSDH and measurement of the postoperative subdural space, which is intuitive, relevant, and rapidly measurable. Patients who score higher on the scale are at greater risk of recurrence and could benefit from adjuvant treatment and more intensive surveillance.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Altaf I, Shams S, Vohra AH. Radiolological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci. 2018. 34: 194-7
2. Amoo M, O’Cearbhaill RM, McHugh P, Henry J, O’Byrne K, Ben Husien M. Derivation of a clinical score for prediction of recurrence following evacuation of chronic subdural hematoma: A retrospective cohort study at a national referral centre. World Neurosurg. 2021. 154: e743-53
3. Andersen-Ranberg NC, Debrabant B, Poulsen FR, Bergholt B, Hundsholt T, Fugleholm K. The Danish chronic subdural hematoma study-predicting recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2019. 161: 885-94
4. Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly-a North Wales experience. J R Soc Med. 2002. 95: 290-2
5. Bartek J, Sjåvik K, Kristiansson H, Ståhl F, Fornebo I, Förander P. Predictors of recurrence and complications after chronic subdural hematoma surgery: A population-based study. World Neurosurg. 2017. 106: 609-14
6. Caetano R, Gruenewald P, Vaeth PA, Canino G. DSM-5 alcohol use disorder severity in Puerto Rico: Prevalence, criteria profile, and correlates. Alcohol Clin Exp Res. 2018. 42: 378-86
7. Chen FM, Wang K, Xu KL, Wang L, Zhan TX, Cheng F. Predictors of acute intracranial hemorrhage and recurrence of chronic subdural hematoma following burr hole drainage. BMC Neurol. 2020. 20: 92
8. Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012. 154: 1541-8
9. Clayton P.editors. CUTPT: Stata Module for Empirical Estimation of Cutpoint for a Diagnostic Test. Statistical Software Components. United States: Boston College Department of Economics; 2013. p.
10. El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000. 11: 553-67
11. Escosa Baé M, Wessling H, Salca HC, de Las Heras Echeverría P. Use of twist-drill craniostomy with drain in evacuation of chronic subdural hematomas: Independent predictors of recurrence. Acta Neurochir (Wien). 2011. 153: 1097-103
12. Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir (Wien). 1975. 32: 247-50
13. Honda M, Maeda H. Intraoperative hematoma volume can predict chronic subdural hematoma recurrence. Surg Neurol Int. 2021. 12: 232
14. Hsieh CT, Su IC, Hsu SK, Huang CT, Lian FJ, Chang CJ. Chronic subdural hematoma: Differences between unilateral and bilateral occurrence. J Clin Neurosci. 2016. 34: 252-8
15. Ihab Z. Pneumocephalus after surgical evacuation of chronic subdural hematoma: Is it a serious complication?. Asian J Neurosurg. 2012. 7: 66-74
16. Jack A, O’Kelly C, McDougall C, Findlay JM. Predicting recurrence after chronic subdural haematoma drainage. Can J Neurol Sci. 2015. 42: 34-9
17. Jung YG, Jung NY, Kim E. Independent predictors for recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2015. 57: 266-70
18. Kanazawa T, Takahashi S, Minami Y, Jinzaki M, Toda M, Yoshida K. Prediction of postoperative recurrence of chronic subdural hematoma using quantitative volumetric analysis in conjunction with computed tomography texture analysis. J Clin Neurosci. 2020. 72: 270-6
19. Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas. No Shinkei Geka. 2011. 39: 1149-53
20. Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: Present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992. 32: 207-9
21. Leroy HA, Aboukaïs R, Reyns N, Bourgeois P, Labreuche J, Duhamel A. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci. 2015. 22: 1895-900
22. Lindvall P, Koskinen LO. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. J Clin Neurosci. 2009. 16: 1287-90
23. Liu X. Classification accuracy and cut point selection. Stat Med. 2012. 31: 2676-86
24. Markwalder TM. Chronic subdural hematomas: A review. J Neurosurg. 1981. 54: 637-45
25. Miah IP, Tank Y, Rosendaal FR, Peul WC, Dammers R, Lingsma HF. Radiological prognostic factors of chronic subdural hematoma recurrence: A systematic review and meta-analysis. Neuroradiology. 2021. 63: 27-40
26. Ohba S, Kinoshita Y, Nakagawa T, Murakami H. The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev. 2013. 36: 145-9
27. Palvanen M, Kannus P, Piirtola M, Niemi S, Parkkari J, Järvinen M. Effectiveness of the Chaos Falls Clinic in preventing falls and injuries of home-dwelling older adults: A randomised controlled trial. Injury. 2014. 45: 265-71
28. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019. 132: 1147-57
29. Ridwan S, Bohrer AM, Grote A, Simon M. Surgical treatment of chronic subdural hematoma: Predicting recurrence and cure. World Neurosurg. 2019. 128: e1010-23
30. Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; Part 5 receiver operating curve and area under the curve. Emerg (Tehran). 2016. 4: 111-3
31. Santarius T, Hutchinson PJ. Chronic subdural haematoma: Time to rationalize treatment?. Br J Neurosurg. 2004. 18: 328-32
32. Schwarz F, Loos F, Dünisch P, Sakr Y, Safatli DA, Kalff R. Risk factors for reoperation after initial burr hole trephination in chronic subdural hematomas. Clin Neurol Neurosurg. 2015. 138: 66-71
33. Shen J, Xin W, Li Q, Gao Y, Zhang J. A grading system for the prediction of unilateral chronic subdural hematoma recurrence after initial single burr hole evacuation. Risk Manag Healthc Policy. 2019. 12: 179-88
34. Shen J, Yuan L, Ge R, Wang Q, Zhou W, Jiang XC. Clinical and radiological factors predicting recurrence of chronic subdural hematoma: A retrospective cohort study. Injury. 2019. 50: 1634-40
35. Sloan T. The incidence, volume, absorption, and timing of supratentorial pneumocephalus during posterior fossa neurosurgery conducted in the sitting position. J Neurosurg Anesthesiol. 2010. 22: 59-66
36. Song DH, Kim YS, Chun HJ, Yi HJ, Bak KH, Ko Y. The predicting factors for recurrence of chronic subdural hematoma treated with burr hole and drainage. Korean J Neurotrauma. 2014. 10: 41-8
37. Stanišić M, Hald J, Rasmussen IA, Pripp AH, Ivanović J, Kolstad F. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: A prospective study of 107 operated patients. Acta Neurochir (Wien). 2013. 155: 323-33
38. Stanisic M, Lund-Johansen M, Mahesparan R. Treatment of chronic subdural hematoma by burr-hole craniostomy in adults: Influence of some factors on postoperative recurrence. Acta Neurochir (Wien). 2005. 147: 1249-56
39. Stanišić M, Pripp AH. A reliable grading system for prediction of chronic subdural hematoma recurrence requiring reoperation after initial burr-hole surgery. Neurosurgery. 2017. 81: 752-60
40. Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001. 54: 774-81
41. Suero Molina E, Borscheid L, Freistühler M, Zawy Alsofy S, Stummer W, Schipmann S. Risk-assessment in chronic subdural hematoma evaluated in 148 patients-a score for predicting recurrence. Clin Neurol Neurosurg. 2020. 195: 106020
42. Uda H, Nagm A, Ichinose T, Onishi Y, Yoshimura M, Tsuruno T. Burr hole drainage without irrigation for chronic subdural hematoma. Surg Neurol Int. 2020. 11: 89
43. Won SY, Dubinski D, Eibach M, Gessler F, Herrmann E, Keil F. External validation and modification of the Oslo grading system for prediction of postoperative recurrence of chronic subdural hematoma. Neurosurg Rev. 2021. 44: 961-70
44. Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003. 98: 1217-21
45. Yang AI, Balser DS, Mikheev A, Offen S, Huang JH, Babb J. Cerebral atrophy is associated with development of chronic subdural haematoma. Brain Inj. 2012. 26: 1731-6
46. You CG, Zheng XS. Postoperative pneumocephalus increases the recurrence rate of chronic subdural hematoma. Clin Neurol Neurosurg. 2018. 166: 56-60