- Department of Radiation Oncology University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States.
- Department of Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States.
Correspondence Address:
Fen Xia, M.D., Ph.D., Chair and Professor, Department of Radiation Oncology, University of Arkansas for Medical Sciences, UAMS Winthrop P. Rockefeller Cancer Institute. Little Rock, AR 72205, USA.
DOI:10.25259/SNI_589_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Arpan V. Prabhu1, Madison Lee2, Edvaldo Galhardo1, Madison Newkirk1, Analiz Rodriguez2, Fen Xia1. Pulsed reduced dose-rate radiotherapy for previously irradiated tumors in the brain and spine. 14-Jun-2021;12:280
How to cite this URL: Arpan V. Prabhu1, Madison Lee2, Edvaldo Galhardo1, Madison Newkirk1, Analiz Rodriguez2, Fen Xia1. Pulsed reduced dose-rate radiotherapy for previously irradiated tumors in the brain and spine. 14-Jun-2021;12:280. Available from: https://surgicalneurologyint.com/surgicalint-articles/10887/
Abstract
Background: Patients with unresectable locoregional cancer recurrences have limited management options. Reirradiation increases the risk of toxicity, particularly when perilesional dose-volume constraints are exceeded. We present and discuss two cases of previously irradiated tumors in the central nervous system (CNS) that was reirradiated using the pulsed reduced dose-rate radiotherapy (PRDR) technique.
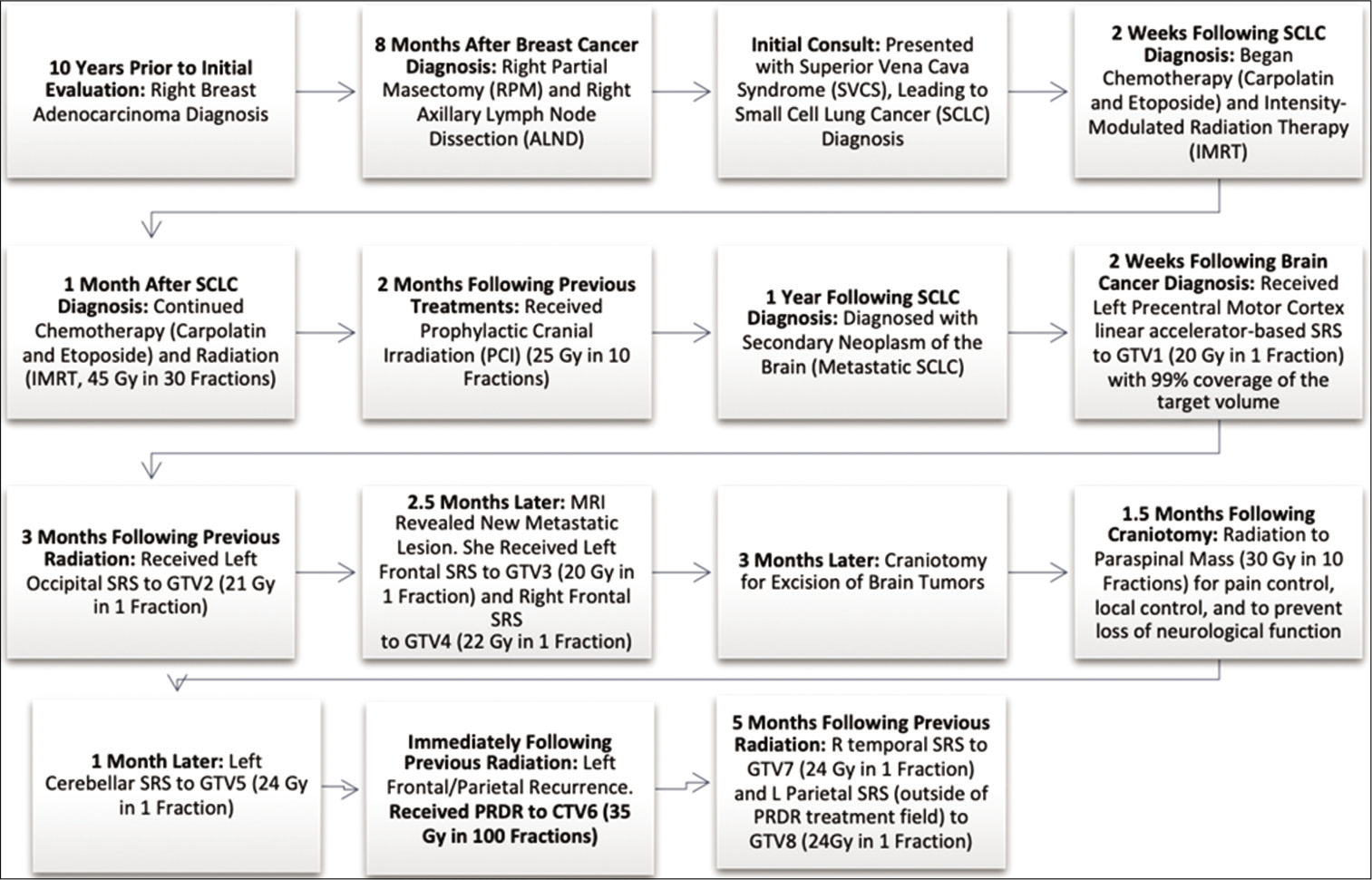
Case Description: A 58-year-old female with a history of metastatic small cell lung cancer to the brain status post multiple rounds of radiation and chemotherapy presented with increasing weakness in her right arm and leg. Magnetic resonance imaging (MRI) revealed a growly peripherally enhancing 1.2 cm mass in the left precentral gyrus that had previously received prophylactic cranial irradiation and stereotactic radiosurgery. The patient was re-irradiated with 35 Gy in 100 fractions over 3 weeks, using PRDR with improved motor function at 3-month follow-up. A 41-year-old male with recurrent glioblastoma of the thoracic spinal cord presented with worsening neurological symptoms, including inability to ambulate due to bilateral leg weakness, causing wheelchair use. MRI thoracic spine revealed a recurrent thoracic lesion 2.2 × 1 × 0.8 cm. In addition to chronic chemotherapy, the patient was retreated palliatively in the same area at 50 Gy in 250 fractions, over 6 weeks, using PRDR. The treated lesion was stable on follow-up imaging, and the patient was able to walk with the assistance of a walker.
Conclusion: In our two cases, PRDR proved effective in the treatment of recurrent malignant CNS tumors that were previously irradiated. Prospective studies are needed to delineate the efficacy and toxicity of PRDR.
Keywords: Glioblastoma multiforme, Metastatic cancer, Pulsed reduced dose-rate radiotherapy, Reirradiation, Small cell lung cancer
INTRODUCTION
Patients with unresectable locoregional recurrence of their cancer have management options, including supportive care only, chemotherapy alone, or re-irradiation with or without concurrent chemotherapy.[
Pulsed reduced dose-rate radiotherapy (PRDR) is a relatively novel radiation technique that was developed at the University of Wisconsin to treat recurrent malignant lesions.[
In this manuscript, we describe two cases in which the CNS tumor is located within the eloquent brain and the intramedullary spine, respectively, not allowing for safe surgical resection. Both patients had clinical neurologic improvement, indicating that PRDR is a reasonable approach even in locations where toxicity can cause potential significant neurologic deficits.
CLINICAL PRESENTATION
Case 1
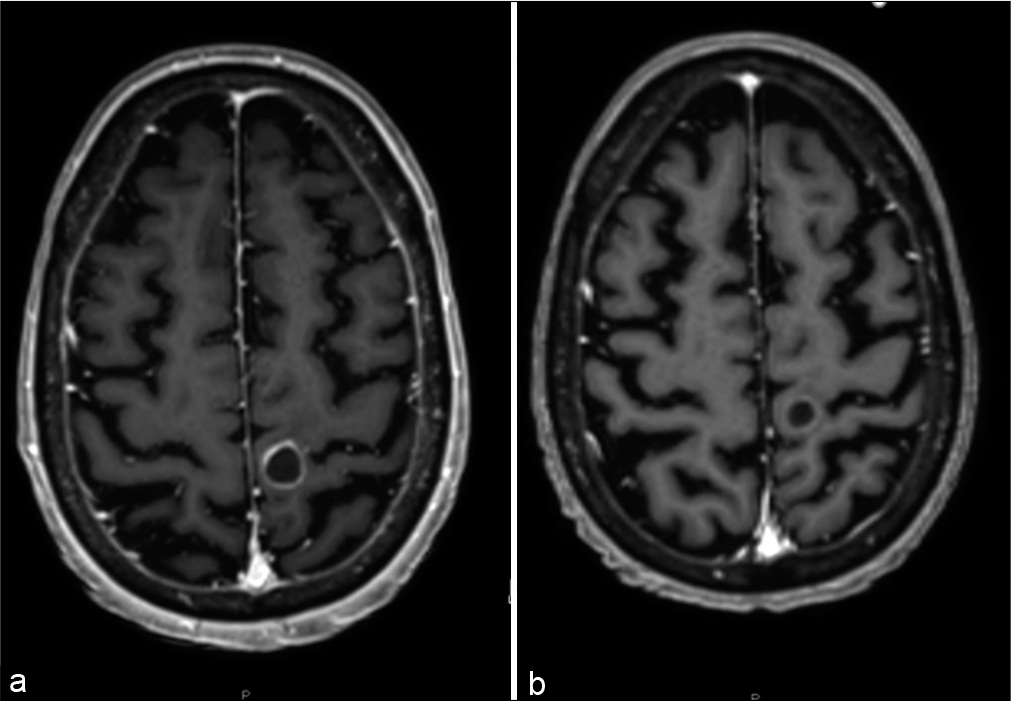
A 58-year-old female with a history of metastatic small cell lung cancer (SCLC) with known recurrent brain metastases and bone metastases status post multiple rounds of radiation and chemotherapy treatments presented with weakness in her right arm and leg, lethargy, and confusion over 1 week. Magnetic resonance imaging (MRI) revealed a growing peripherally enhancing 1.2 cm mass (0.8 cm 2 months prior) in the left precentral gyrus that had previously received linear-accelerator-based stereotactic radiosurgery (SRS) to 20 Gy 9 months prior (with 99% coverage of the target volume), a new left cerebellar 0.4 cm lesion, and a growing left posterior temporal/medial occipital 0.6 cm lesion (0.4 cm 2 months prior) [
The patient had a complicated oncological treatment history, including a previous diagnosis of right breast adenocarcinoma status post breast-conserving surgery and adjuvant chemoradiation, concurrent definitive chemoradiation for her originally diagnosed limited-stage SCLC, prophylactic cranial irradiation (PCI), and SRS to multiple brain metastases. [
Following discussion at a multidisciplinary tumor board, the patient underwent craniotomy and resection of her left precentral gyrus lesion, with pathology demonstrating metastatic SCLC. Given that the target area had previously received radiation therapy with PCI and SRS, the patient was given 35 Gy in 100 fractions over the course of 3 weeks, using PRDR for re-irradiation treatment at an apparent dose rate of 0.178 Gy/min for a cumulative dose of 80 Gy. TomoHDA™ Planning Station Version 5.1.1.6 (Accuray Inc., Sunnyvale, CA, USA) was used [
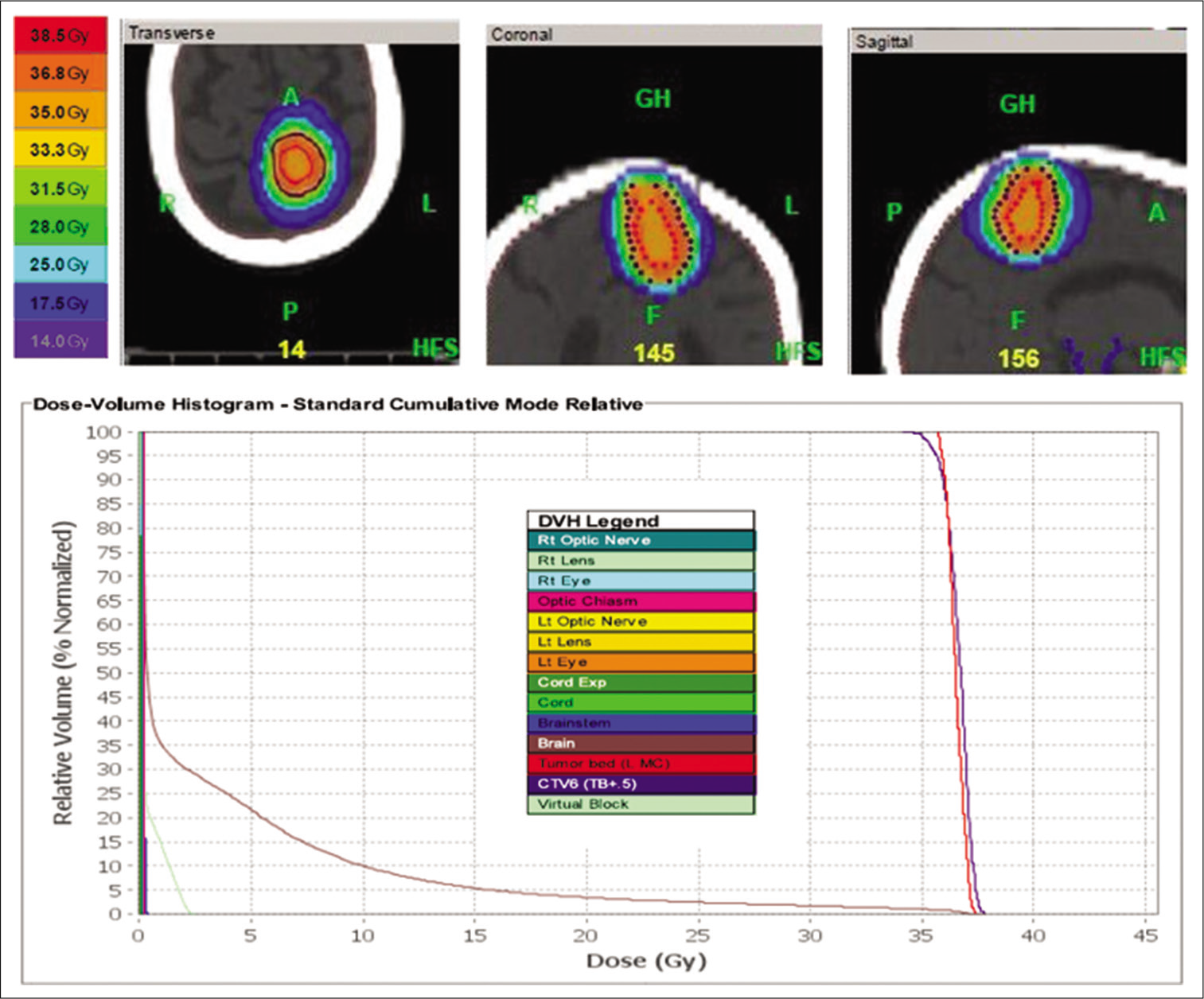
Figure 3:
Left precentral motor cortex lesion re-treatment plan with dose-volume histogram. This patient was re-treated with 35 Gy in 100 fractions, over the course of 3 weeks, to her surgical cavity of her left precentral gyrus lesion. Pulsed reduced dose-rate radiotherapy was used with TomoHDA™ Planning Station Version 5.1.1.6, and 97% of the target volume received 35 Gy. The colors in the top of the figure correspond to isodose distributions, with orange corresponding to the volume receiving 35Gy (100% dose area). The dose-volume histogram relates radiation dose to tissue volume and includes both the target structure as well as organs at risk.
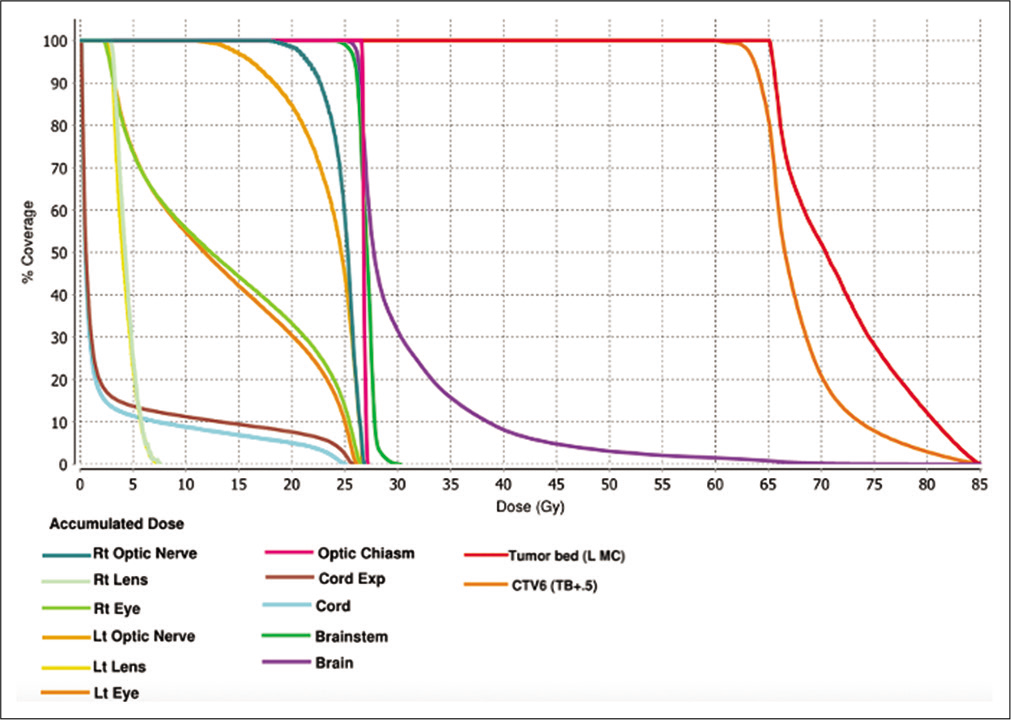
Figure 4:
Comprehensive dose-volume histogram based on a summation of the patient’s previous radiation treatments detailed in
At follow-up in 3 months after PRDR treatment, the patient noted improved motor function of her right hand and foot. MRI brain showed improvement with marked reduction in enhancement in the lesions in the left precentral gyrus, left occipital, and left cerebellar lesions. The patient was started on palliative carboplatin and etoposide. In 5 months following the PRDR treatment, the patient maintained good motor function of her right hand but developed progression of disease with two new 3 mm brain metastases in the right temporal and left parietal regions that were outside the previously treated PRDR field. She received 24 Gy SRS to those lesions. She then developed progression in the mediastinum and retroperitoneum, although there was no evidence of recurrence within the PRDR-treated radiation field. The patient was started on palliative pembrolizumab but in the coming months had a gradual decrease in her overall performance status with increased altered mental status and hospitalizations. After discussion with neurosurgery and palliative care, the patient switched her care to home hospice, which she continues with 14 years elapsing since her initial diagnosis.
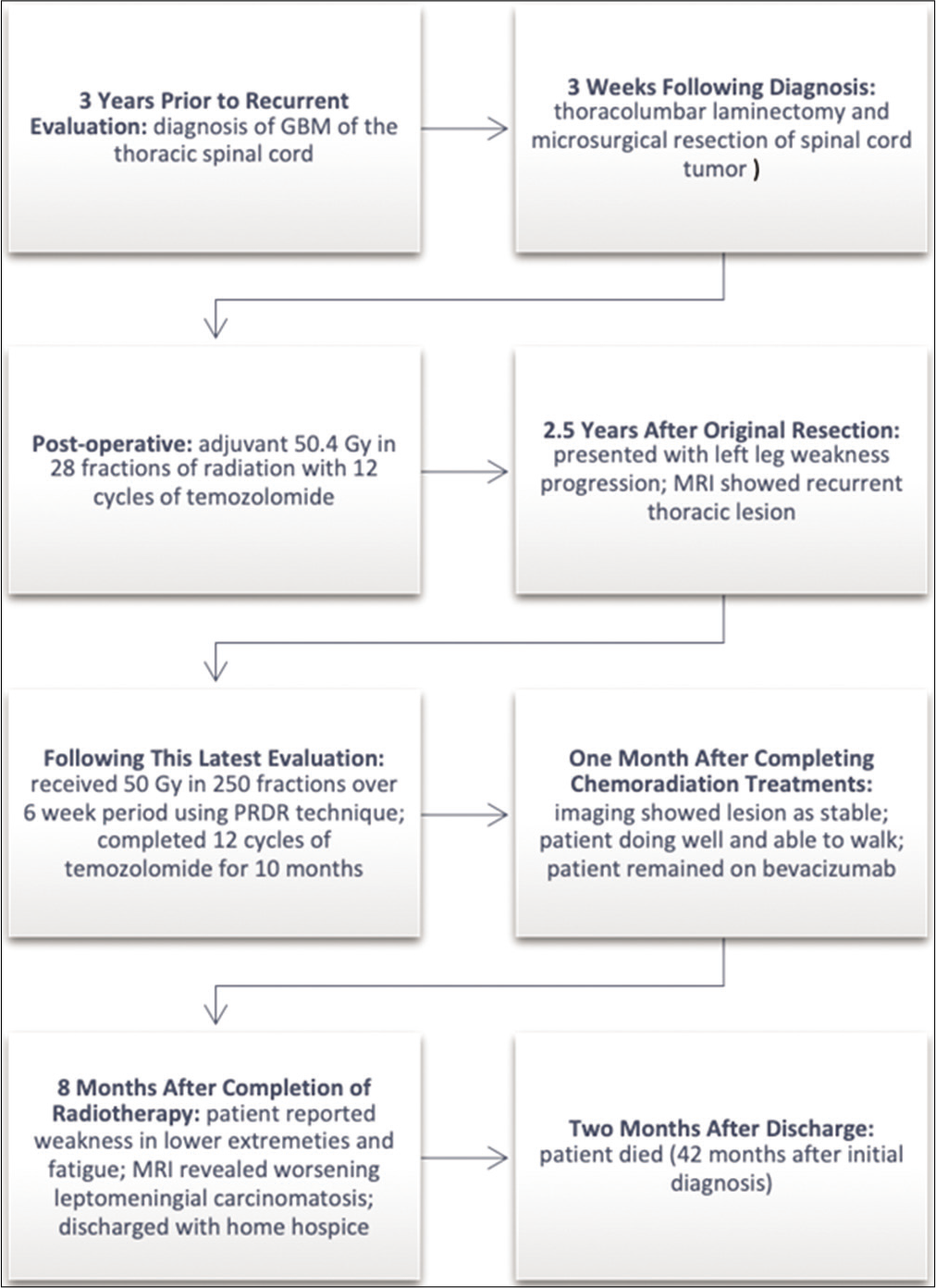
Case 2
[
Two and a half years after the original resection, his left leg weakness progressed, and an MRI of the thoracic spine showed definite tumor progression. Additional chemotherapy with temozolomide, Avastin, isotretinoin, and lomustine did not slow the progression of the tumor. At the time of evaluation, the patient reported a 6-month history of a 20-pound weight loss, worsening bilateral leg weakness, numbness and tingling in both legs, erectile dysfunction, inability to ambulate with both legs causing wheelchair use, increased urinary frequency, and occasional difficulty holding bowel movements. He denied any pain. His ECOG performance status was 3.
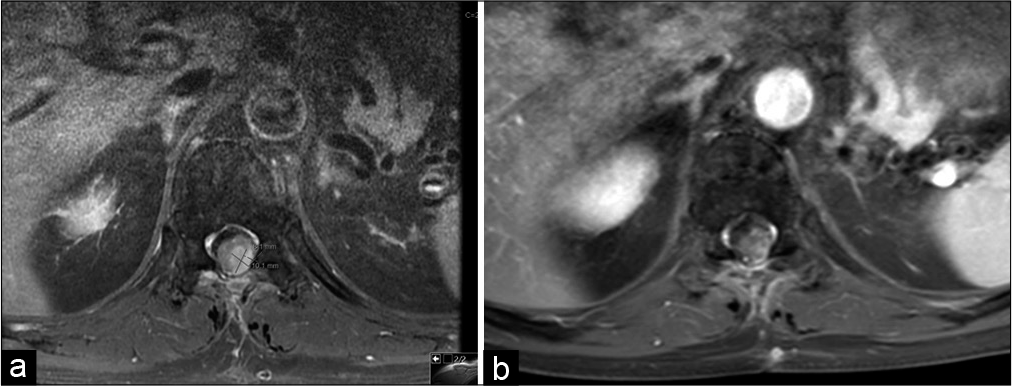
MRI thoracic spine revealed a recurrent thoracic lesion 2.2 × 1 × 0.8 cm [
Figure 7:
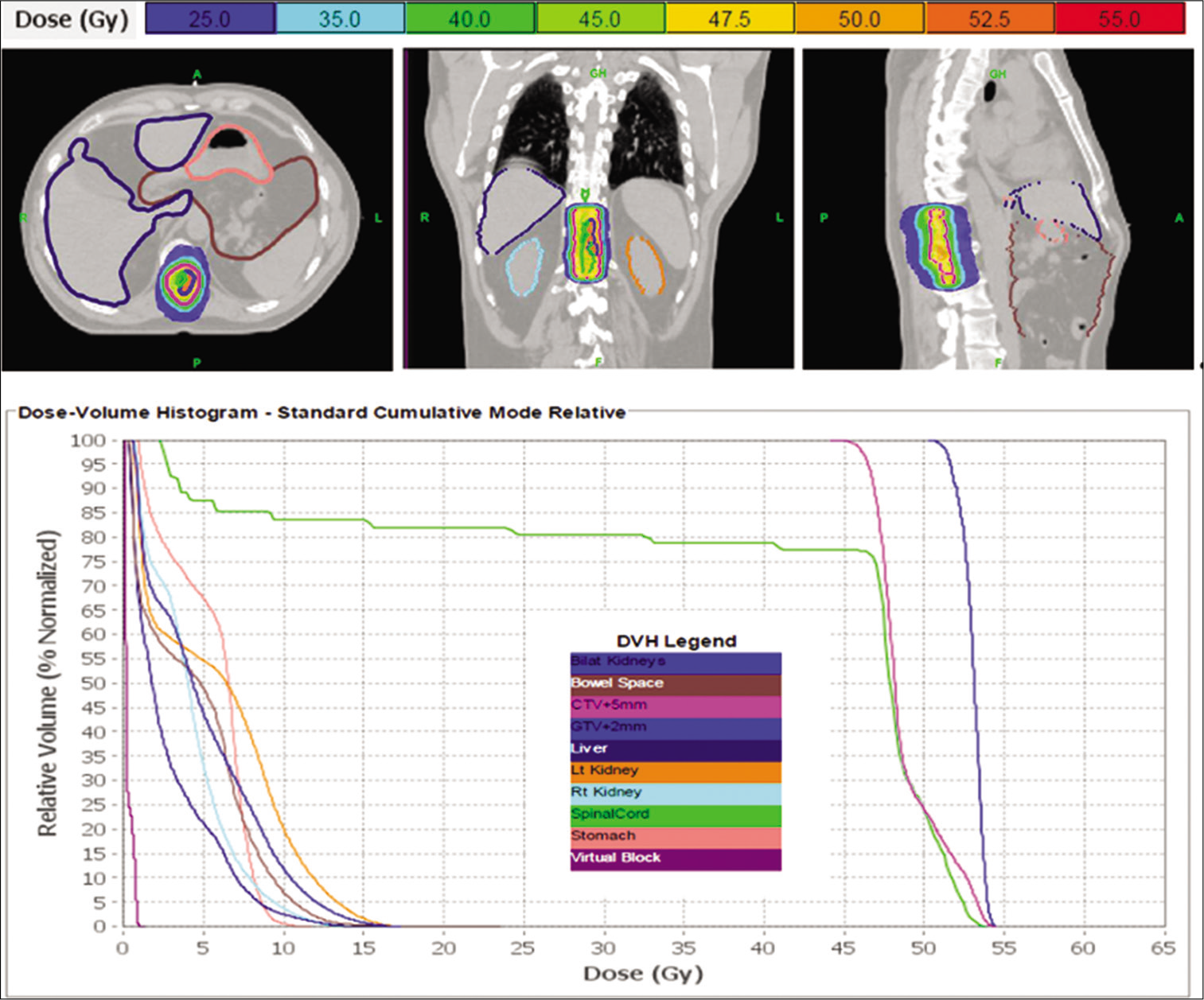
Thoracic spinal glioblastoma multiforme re-treatment plan with dose-volume histogram. This patient was re-treated with 50 Gy in 250 fractions, over the course of 6 weeks, to his recurrent thoracic lesion. Pulsed reduced dose-rate radiotherapy was used with TomoHDATM Planning Station Version 5.1.1.6, and 99% of the target volume received 50 Gy. The colors in the top of the figure correspond to isodose distributions for the treatment target in the thoracic spine, with orange corresponding to the volume receiving 50 Gy (100% dose area). Organs at risk are delineated in the top of the figure as well. The dose-volume histogram relates radiation dose to tissue volume and includes both the target structure as well as organs at risk.
[
Figure 8:
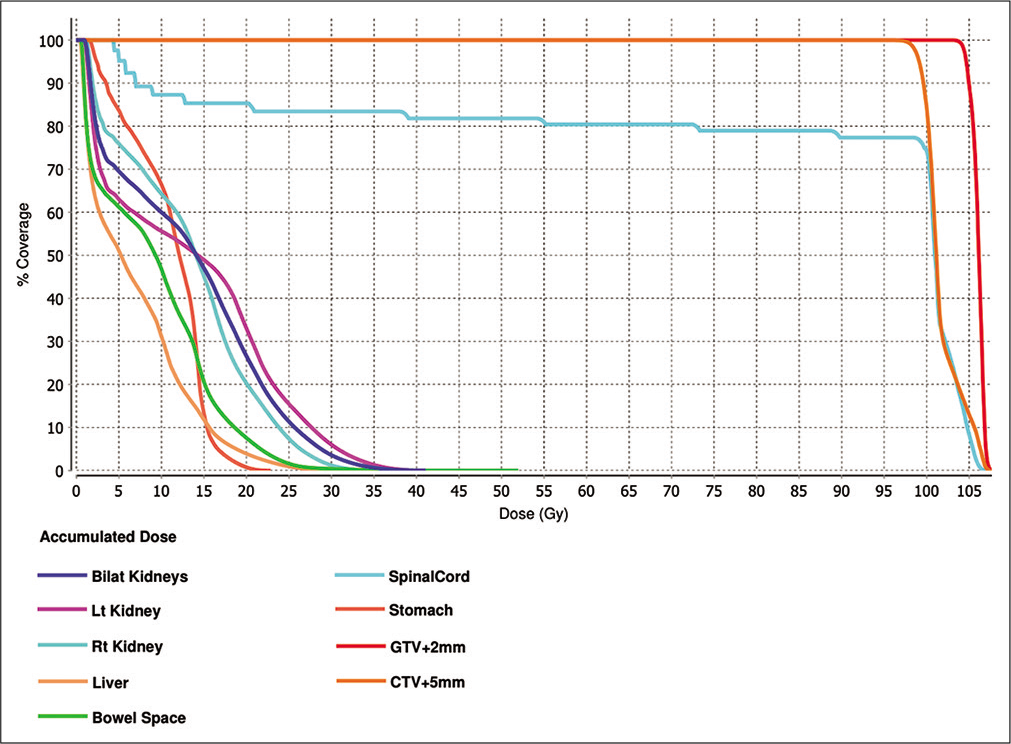
Comprehensive dose-volume histogram based on a summation of the patient’s previous radiation treatments detailed in
Imaging showed the lesion as stable at the 1-month follow-up appointment. He was able to walk with the assistance of a walker, which was an improvement from previously being bound by a wheelchair. Overall, the patient did not develop significant acute radiation side effects and reported to be very satisfied with his response to treatment.
Eight months after completion of radiation treatment, the patient remained on bevacizumab under the care of his oncologist. The patient reported progressive weakness in his lower extremities and ongoing fatigue. MRI of the spine revealed worsening leptomeningeal carcinomatosis, and the patient was discharged with home hospice. He died 2 months later, about 42 months after his initial diagnosis.
DISCUSSION
For patients with CNS cancers that have been previously irradiated, increased risks of radiation-induced complications discourage radiation oncologists from pursuing re-irradiation. Re-irradiation is difficult due to normal tissue tolerances. Normal tissues that receive reirradiation may experience more severe acute and late radiation complications compared with the initial course. Historically, there has been a lack of enthusiasm for reirradiation based on the assumption that unsuccessful initial radiation begets poor results with salvage irradiation. In addition to nervous tissue toxicity, the total radiation dose, volume, interval between radiation treatments, and performance status of the patient are taken into account when deciding the feasibility of reirradiation.[
PRDR is a radiation technique that has been radiobiologically derived to exploit the differences between normal neural tissue and glial neoplasms for repair of radiation damage. It breaks treatment into a number of subfractions, delivering radiation pulses of 0.2 Gy separated by 3 min intervals, creating an apparent low dose rate treatment regime.[
PRDR has been used in a variety of cancers, including recurrent high-grade gliomas,[
The exact mechanism of PRDR is unknown. [
There are limitations to the PRDR technique. Treatment time is longer, potentially decreasing the overall dose delivery accuracy if the target deviates from its original position. Daily 3D cone beam computed tomography scans and treatment fixation devices were used for our patients, including a head and neck mask and immobilization cushion devices. Other reports suggest performing two megavoltage computed tomography scans before the first and fourth subfractions to determine if there is any trend of target deviation.[
PRDR is palliative in nature yet may offer sustained tumor control, prolongation of survival, and symptom relief. Indeed, the two patients presented here improved clinically until there was disease progression.
CONCLUSION
In our two cases, PRDR proved effective in the treatment of recurrent malignant CNS tumors that were previously irradiated. Prospective studies are needed to delineate the efficacy and toxicity of PRDR.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adkison JB, Tome W, Seo S, Richards GM, Robins HI, Rassmussen K. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011. 79: 835-41
2. Almahariq MF, Quinn TJ, Arden JD, Roskos PT, Wilson GD, Marples B. Pulsed radiation therapy for the treatment of newly diagnosed glioblastoma. Neuro Oncol. 2020. 23: 447-56
3. Bovi JA, Prah MA, Retzlaff AA, Schmainda KM, Connelly JM, Rand SD. Pulsed reduced dose rate radiotherapy in conjunction with bevacizumab or bevacizumab alone in recurrent high-grade glioma: Survival outcomes. Int J Radiat Oncol Biol Phys. 2020. 108: 979-86
4. Cannon GM, Tome WA, Robins HI, Howard SP. Pulsed reduced dose-rate radiotherapy: Case report: A novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J Neurooncol. 2007. 83: 307-11
5. Hall EJ, Brenner DJ. The dose-rate effect revisited: Radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys. 1991. 21: 1403-14
6. Hall EJ, Giaccia AJ.editors. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p.
7. Harney J, Short SC, Shah N, Joiner M, Saunders MI. Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys. 2004. 59: 1190-5
8. Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001. 49: 379-89
9. Khan M, Zhao Z, Arooj S, Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: A systematic review and meta-analysis. BMC Cancer. 2021. 21: 167
10. Lambin P, Coco-Martin J, Legal JD, Begg AC, Parmentier C, Joiner MC. Intrinsic radiosensitivity and chromosome aberration analysis using fluorescence in situ hybridization in cells of two human tumor cell lines. Radiat Res. 1994. 138: S40-3
11. Lambin P, Fertil B, Malaise EP, Joiner MC. Multiphasic survival curves for cells of human tumor cell lines: Induced repair or hypersensitive subpopulation?. Radiat Res. 1994. 138: S32-6
12. Lambin P, Malaise EP, Joiner MC. The effect of very low radiation doses on the human bladder carcinoma cell line RT112. Radiother Oncol. 1994. 32: 63-72
13. Lambin P, Malaise EP, Joiner MC. Might intrinsic radioresistance of human tumour cells be induced by radiation?. Int J Radiat Biol. 1996. 69: 279-90
14. Lambin P, Marples B, Fertil B, Malaise EP, Joiner MC. Hypersensitivity of a human tumour cell line to very low radiation doses. Int J Radiat Biol. 1993. 63: 639-50
15. Li GH, Liu Y, Tang JL, Zhang D, Zhou P, Yang DQ. Pulsed reduced dose-rate radiotherapy as re-irradiation for brain metastasis in a patient with lung squamous-celled carcinoma. Jpn J Clin Oncol. 2012. 42: 856-60
16. Li GH, Zhu B, Yang F, Ma CK, Yang DQ. Use of cetuximab in combination with pulsed reduced dose-rate radiotherapy in a patient with recurrence of nasopharyngeal carcinoma in the neck. Exp Ther Med. 2012. 3: 869-72
17. Li J, Lang J, Wang P, Kang S, Lin MH, Chen X. Intensity-modulated radiation therapy for pancreatic and prostate cancer using pulsed low-dose rate delivery techniques. Med Dosim. 2014. 39: 330-6
18. Lin PS, Wu A. Not all 2 Gray radiation prescriptions are equivalent: Cytotoxic effect depends on delivery sequences of partial fractionated doses. Int J Radiat Oncol Biol Phys. 2005. 63: 536-44
19. Marples B. Is low-dose hyper-radiosensitivity a measure of G2-phase cell radiosensitivity?. Cancer Metastasis Rev. 2004. 23: 197-207
20. Mohindra P, Robins HI, Tome WA, Hayes L, Howard SP. Wide-field pulsed reduced dose rate radiotherapy (PRDR) for recurrent ependymoma in pediatric and young adult patients. Anticancer Res. 2013. 33: 2611-8
21. Murphy ES, Rogacki K, Godley A, Qi P, Reddy CA, Ahluwalia MS. Intensity modulated radiation therapy with pulsed reduced dose rate as a reirradiation strategy for recurrent central nervous system tumors: An institutional series and literature review. Pract Radiat Oncol. 2017. 7: e391-9
22. Nieder C, Andratschke NH, Grosu AL. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016. 36: 4985-95
23. Nieder C, Gaspar LE, Ruysscher D, Guckenberger M, Mehta MP, Rusthoven CG. Repeat reirradiation of the spinal cord: Multi-national expert treatment recommendations. Strahlenther Onkol. 2018. 194: 365-74
24. Richards GM, Tome WA, Robins HI, Stewart JA, Welsh JS, Mahler PA. Pulsed reduced dose-rate radiotherapy: A novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009. 114: 307-13
25. Rogacki KC, Chao ST, Yu J, Godley A, Balagamwala E, Suh JH. Review of pulsed reduced dose rate re-irradiation for recurrent tumors. J Cancer Clin Trials. 2018. 3: 2
26. Rong Y, Paliwal B, Howard SP, Welsh J. Treatment planning for pulsed reduced dose-rate radiotherapy in helical tomotherapy. Int J Radiat Oncol Biol Phys. 2011. 79: 934-42
27. Shen G, Wang YJ, Guan YJ, Dong DP, Yang G, Li D. Relief effect of bevacizumab on severe edema induced by re-irradiation in brain tumor patients. Chin Med J (Engl). 2015. 128: 2126-9
28. Short S, Mayes C, Woodcock M, Johns H, Joiner MC. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol. 1999. 75: 847-55
29. Short SC, Mitchell SA, Boulton P, Woodcock M, Joiner MC. The response of human glioma cell lines to low-dose radiation exposure. Int J Radiat Biol. 1999. 75: 1341-8
30. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016. 41: E12
31. van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AM, Vandertop WP. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J Neurooncol. 2017. 135: 183-92
32. Witt JS, Musunuru HB, Bayliss RA, Howard SP. Large volume re-irradiation for recurrent meningioma with pulsed reduced dose rate radiotherapy. J Neurooncol. 2019. 141: 103-9
33. Wouters BG, Sy AM, Skarsgard LD. Low-dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res. 1996. 146: 399-413
34. Zhang P, Wang B, Chen X, Cvetkovic D, Chen L, Lang J. Local tumor control and normal tissue toxicity of pulsed low-dose rate radiotherapy for recurrent lung cancer: An in vivo animal study. Dose Response. 2015. 13: 1559325815588507