- Department of Neurosurgery, St George Hospital, Kogarah, New South Wales, 2217, Australia,
- Conjoint Associate Lecturer, St George Hospital and Sutherland Clinical School, UNSW Medicine, Australia,
- Histopathology Department, Douglass Hanly Moir Pathology (Sonic Healthcare), Macquarie Park, NSW, 2113, Australia,
- Discipline of Pathology, Faculty of Medicine, Health and Human Sciences, Macquarie University, NSW, 2109, Australia,
- Sydney Medical School, The University of Sydney, Sydney, 2006, Australia,
- Cancer Diagnosis and Pathology Group, Kolling Institute of Medical Research, St Leonards, NSW, 2065, Australia,
- Conjoint Associate Professor, St George and Sutherland Clinical School, UNSW Medicine, Australia.
Correspondence Address:
Cher Shui, Department of Neurosurgery, St George Hospital, Kogarah, New South Wales, 2217, Conjoint Associate Lecturer, St George Hospital and Sutherland Clinical School, UNSW Medicine, Australia.
DOI:10.25259/SNI_653_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Cher Shui1,2, John Turchini3,4,5,6, Mark Davies1,7. Purely extradural spinal meningioma: A case report and literature review. 30-Aug-2021;12:431
How to cite this URL: Cher Shui1,2, John Turchini3,4,5,6, Mark Davies1,7. Purely extradural spinal meningioma: A case report and literature review. 30-Aug-2021;12:431. Available from: https://surgicalneurologyint.com/surgicalint-articles/11078/
Abstract
Background: Purely extradural spinal meningiomas (ESMs) are exceptionally rare and are often incorrectly diagnosed as metastases, hematological malignancies, or schwannomas. Here, we report a 66-year-old female who presented with an isolated extradural ESM.
Case Description: A 66-year-old female presented with a 2.5-year history of a progressive paraparesis (i.e. T7 level) associated with a T5 sensory level. The MR showed a heterogeneously enhancing lesion circumferentially involving the spinal cord from T3 to T5, with left-sided T4/5 foraminal extension. Following a Simpson Grade 2 resection, the patient rapidly recovered full neurological function.
Conclusion: The vast majority of the rarely encountered purely ESM are benign. Although gross total resection is optimal, additional adjunctive treatments are available for those treated with subtotal resections.
Keywords: Gross total resection, Purely extradural meningioma, Spinal meningioma
INTRODUCTION
Purely extradural spinal meningiomas (ESMs) are extremely rare and are, therefore, often misdiagnosed as metastases or hematological malignancies. Here, we present a 66-year-old female whose dumbbell-shaped, purely extradural thoracic meningioma was effectively excised, resulting in the resolution of her preoperative paraparesis.
CASE REPORT
History and presentation
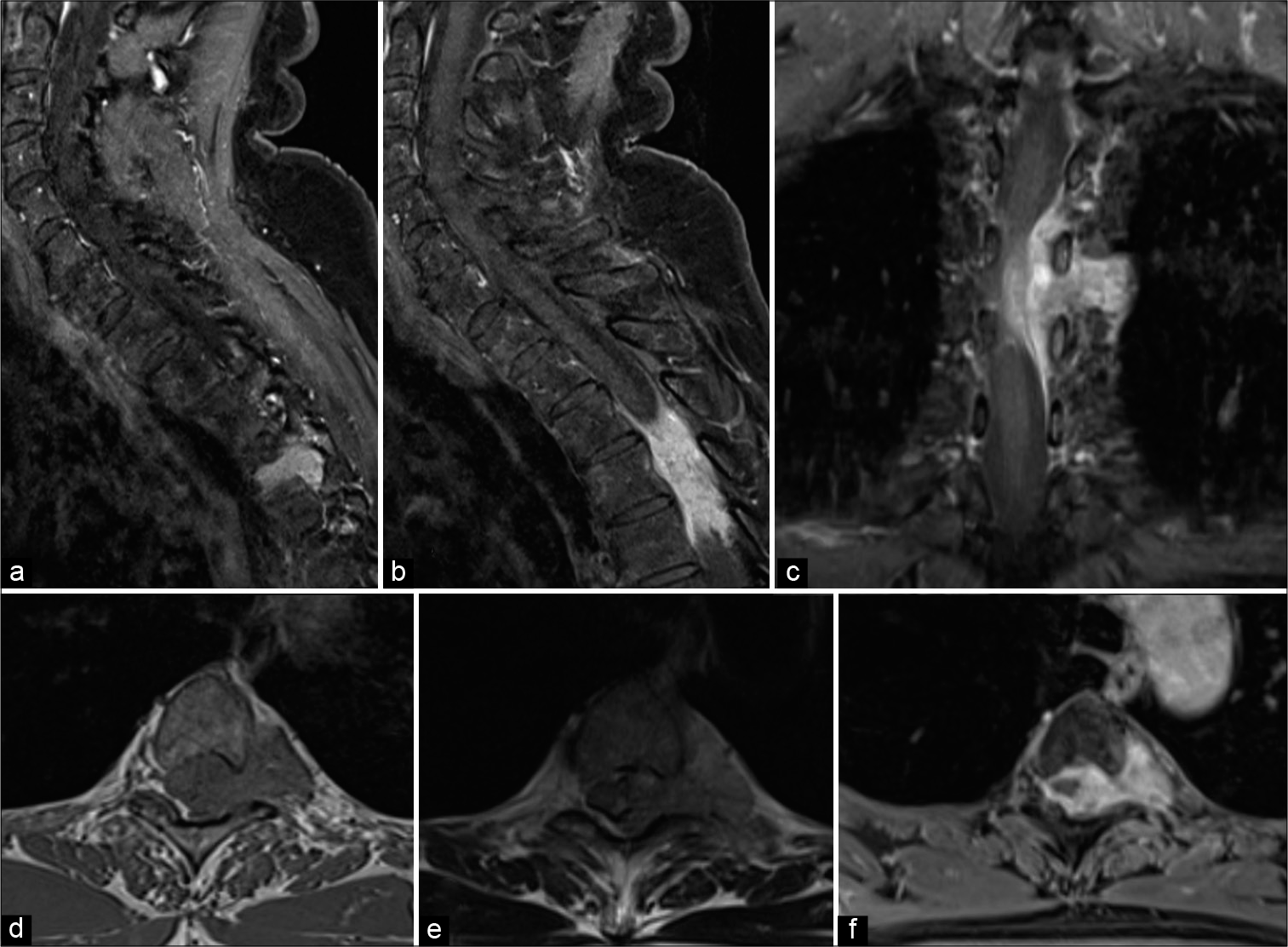
A 66-year-old female presented with a 2.5-year history of a progressive thoracic paraparesis (i.e. relative sensory level T5) without sphincter involvement. The spinal MRI performed with and without intravenous gadolinium demonstrated a dumbbell-shaped, heterogeneously enhancing lesion almost encircling the spinal cord from T3 to T5 plus left-sided foraminal T4/5 extension. Maximal compression was present at the T4 level resulting in rightward cord displacement and increased T2 signal in the cord. The CT of the chest, abdomen, and pelvis did not reveal any additional extra-spinal malignancy or metastases. Further, the calcification seen on CT, and lack of bony destruction was consistent with a benign lesion [
Figure 1:
Preoperative MR images of the lesion measuring 28 mm × 43 mm × 21 mm. (a and b) Sagittal T1-weighted images with gadolinium enhancement. Avid, heterogeneously enhancing lesion extending from T3 to T5. (c) Coronal T1-weighted images demonstrating tumor displacing the cord to the right with cord signal change. (d) Axial T1-weighted image. Lesion demonstrating homogenous low signal. (e) Axial T2-weighted image. (f) Axial T1-weighted image.
Presurgical spinal angiogram embolization
When preoperative spinal angiography was performed, it demonstrated that the left superior intercostal trunk was the main feeding artery (i.e. originated from the T5 level giving off the third, fourth, and fifth intercostal arteries) to the tumor. This was safely embolized.
Surgery
Surgery included a T3–T5 laminectomy, unroofing of the left T4/5 foramen, and resection of the head of the T4 rib. Tumor was dissected off the dura between T3 and T5, including the left T5 nerve root, following which a T3–T5 fusion was performed (i.e., pedicle/screws). A near-complete resection was achieved, leaving only a thin film of tumor extending rostrally in the left paravertebral gutter near the 3rd rib head.
Histological analysis
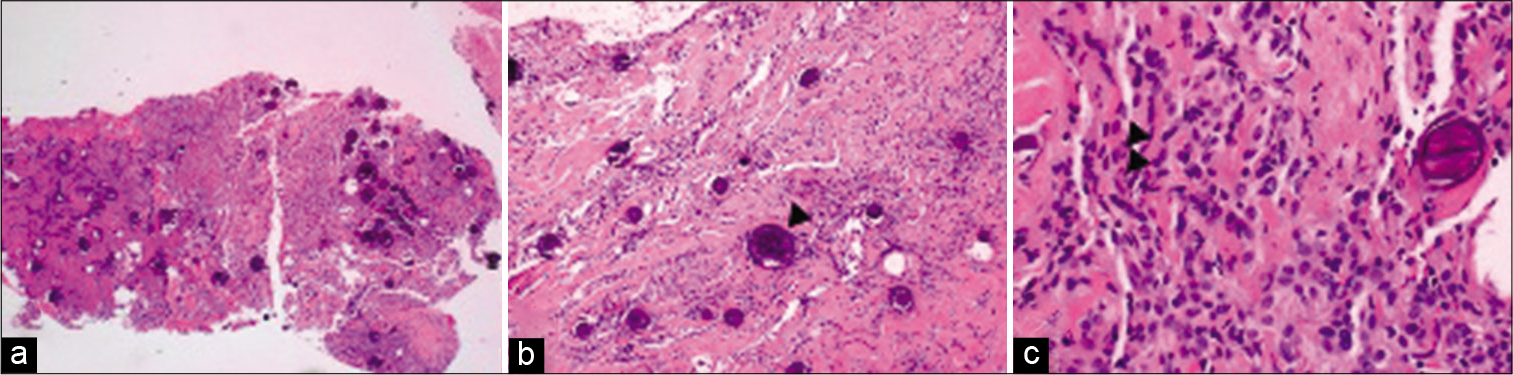
The intraoperative frozen section report was consistent with a WHO Grade I meningioma. The lesion was mengiothelial in morphology (i.e., whorling architecture, occasional psammomatous calcification, and intranuclear inclusions) and infiltrated through a fibrous dura and into the surrounding fibroadipose tissue. There was less than 1 mitotic figure seen in any 10 high-power fields. The tumor was positive for EMA and PR. The Ki-67 was 2% [
Figure 2:
Photomicrographs of the specimen. (a) Meningioma infiltrating through dura (H&E, ×100). (b) Meningioma infiltrating through fibrous dura with prominent psammomatous calcifications (arrowhead) (H&E, ×200). (c) Intranuclear pseudoinclusions (demonstrated by arrowheads) and typical meningothelial morphology (H&E, ×400).
Postoperative course
Three months postoperatively, the patient fully recovered except for mild residual left-sided T2–T5 numbness. The repeat thoracic MRI showed complete tumor resection.
DISCUSSION AND LITERATURE REVIEW
Epidemiological features
Purely ESMs are exceedingly rare, present in 2.5–5% of spinal meningiomas. A review of the MRI era literature identified 28 cases of purely ESM in adults averaging 47 years of age, with most cases involving the cervical spine (61%), followed by the thoracic spine (54%) and only rarely (i.e. two cases) did they occur in the lumbar spine.
Clinical presentation
Spinal meningiomas are most commonly benign, often with a long clinical history until a radiological diagnosis is made. Most patients present with pain (57%), motor deficits (57%), and sensory deficits (50%), typically over an average of 19 months.[
Diagnostic evaluation and imaging features
MR is the study of choice. Lesions are usually hypointense or isointense on T1 examinations, but demonstrate varied intensities on T2-weighted images (i.e., 32% were hyperintense on T2-weighted images, while 25% were isointense and 14% were hypodense).
Differential diagnoses
The extradural location of these lesions, often with foraminal and paravertebral extension, leads to additional malignant diagnoses including spinal metastases, extradural lymphoma, or a malignant nerve sheath tumor.
Surgical considerations and tumor resection
Resection of spinal extradural meningiomas can be technically difficult and some require a second approach.[
Histopathology and adjuvant therapies
The vast majority (93%) of extradural meningiomas are Grade I meningiomas – most commonly meningothelial (36%) and psammomatous (25%). Only two cases of atypical meningioma occurred extradurally, comprising 7% of the cases identified. Both patients received radiation therapy.[
Long-term outcomes and follow-up
The vast majority of cases of extradural meningiomas in the literature (85%) described marked symptom improvement postoperatively with no evidence of clinical or radiological progression on follow-up (mean follow-up 6 months, range 1–84 months).
CONCLUSION
Surgical management for purely ESMs can lead to excellent outcomes, while subtotal resection may be effectively treated with appropriate adjuvant therapies, such as stereotactic radiosurgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ben Nsir A, Boughamoura M, Mahmoudi H, Kilani M, Hattab N. Uncommon progression of an extradural spinal meningioma. Case Rep Surg. 2014. 2014: 630876
2. Bettaswamy G, Ambesh P, Das KK, Sahu R, Srivastava A, Mehrotra A. Extradural spinal meningioma: Revisiting a rare entity. J Craniovertebr Junction Spine. 2016. 7: 65-8
3. Choi J, Hong HJ, Paik SY. spinal epidural en plaque meningioma misdiagnosed as lymphoma: A case report. J Korean Soc Radiol. 2019. 80: 1235
4. D’Amico A, Napoli M, Cirillo M, D’Arco F, D’Anna G, Caranci F. Imaging of cervical extradural en-plaque meningioma. A case report. Neuroradiol J. 2012. 25: 598-603
5. Dehcordi SR, Ricci A, Chiominto A, de Paulis D, di Vitantonio H, Galzio RJ. Dorsal extradural meningioma: Case report and literature review. Surg Neurol Int. 2016. 7: 76
6. Kale A, Akyol C, Keskin E, Aydoğmuş E, Aydın HA, Barut F. Coexistence of cervico-thoracic extradural en-plaque meningioma with multiple intracranial meningiomas. Neurol Neurochir Pol. 2014. 48: 363-7
7. Lai AL, Salkade PR, Chuah KL, Sitoh YY. Extradural cervical spinal meningioma mimicking malignancy. J Radiol Case Rep. 2018. 12: 1-10
8. Sato N, Sze G. Extradural spinal meningioma: MRI. Neuroradiology. 1997. 39: 450-2