- Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
Correspondence Address:
Michael W. Kortz, Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
DOI:10.25259/SNI_530_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Evan Winograd, Michael W. Kortz, Kevin O. Lillehei. Radiographic pituitary stalk disruption: A rare sequela of secondary empty sella syndrome. 03-Aug-2021;12:385
How to cite this URL: Evan Winograd, Michael W. Kortz, Kevin O. Lillehei. Radiographic pituitary stalk disruption: A rare sequela of secondary empty sella syndrome. 03-Aug-2021;12:385. Available from: https://surgicalneurologyint.com/surgicalint-articles/11019/
Abstract
Background: This two-patient case series describes a rare sequela of postoperative empty sella syndrome (ESS) following transsphenoidal resection of pituitary macroadenomas. This is characterized by progressive hormone dysfunction, diabetes insipidus (DI), and associated MRI evidence of pituitary stalk disruption.
Case Description: This phenomenon was retrospectively evaluated in a review of 2000 pituitary tumor resections performed by a single neurosurgeon (KOL). Chart review was retrospectively conducted to gather data on demographics, pituitary hormone status, tumor characteristics, and management. We identified 2 (0.1%) cases of progressive pituitary endocrine dysfunction occurring in the postoperative period associated with MRI evidence of pituitary stalk disruption within 6 weeks of discharge from the hospital. This was felt to be caused by the rapid descent of the residual normal pituitary gland down to the floor of the postoperative empty sella, causing relatively swift stalk stretching. Both patients developed DI, and one patient demonstrated increased pituitary hormone dysfunction.
Conclusion: This phenomenon is a rare manifestation of postoperative ESS, secondary to surgical resection of a pituitary macroadenoma. We discuss the associated potential risk factors and strategies for avoidance in these two cases. Routine instillation of intrasellar fat in patients at risk is felt to be protective.
Keywords: Empty sella syndrome, Endocrine dysfunction, Pituitary adenoma, Pituitary stalk disruption, Transsphenoidal resection
INTRODUCTION
Empty sella syndrome (ESS) is a condition in which the sella turcica is partially or filled with cerebrospinal fluid (CSF) resulting in downward displacement of the normal pituitary gland.[
We describe two cases of secondary ESS associated with downward displacement of the residual normal pituitary gland, with MRI evidence of pituitary stalk disruption. Both cases were without optic chiasm involvement. In the postoperative period, these two patients exhibited progressive endocrine dysfunction along with the delayed onset of diabetes insipidus (DI). The finding of progressing endocrine dysfunction in the weeks following surgery, with MRI evidence of pituitary stalk disruption is rare, seen in two cases to date at our institution in review of 2000 transsphenoidal tumor resections.
MATERIALS AND METHODS
Study design and eligibility criteria
Patients aged 18 years or older who underwent transsphenoidal surgery (TSS) for a pituitary tumor at our institution from July 2000 to January 2020 were retrospectively identified. In our review, 2000 patients underwent TSS at our institution in the study period. Of this cohort, only 2 (0.10%) demonstrated rapid short-term pituitary endocrinopathy and DI with MRI evidence of pituitary stalk disruption. Case presentations are described below. Data were then retrospectively gathered through electronic health record review, including demographics, laboratory values, radiographs, and operative management. Per independent Colorado Multiple Institutional Review Board guidelines, this study was determined to be exempt.
Pituitary adenoma clinical protocol
At our institution, surgical management for patients with pituitary adenomas is recommended for those presenting with symptoms of mass effect (headache, visual compromise, or endocrinopathy), growth hormone, adrenocorticotropic hormone, or thyroid-stimulating hormone (TSH) hypersecretion, or hyperprolactinemia refractory to medical management. The choice of microscopic or endoscopic TSS depends on surgeon preference, patient anatomy, and tumor characteristics (i.e. cavernous sinus involvement or suprasellar extension). Hormone dysfunction in the postoperative period is managed with the corresponding agent until there is evidence of resolution. Routine follow-up consists of an MRI of the pituitary 3 months postoperatively and then at various intervals thereafter depending on resection rate and the patient’s symptoms.
CASE DESCRIPTION
Case 1
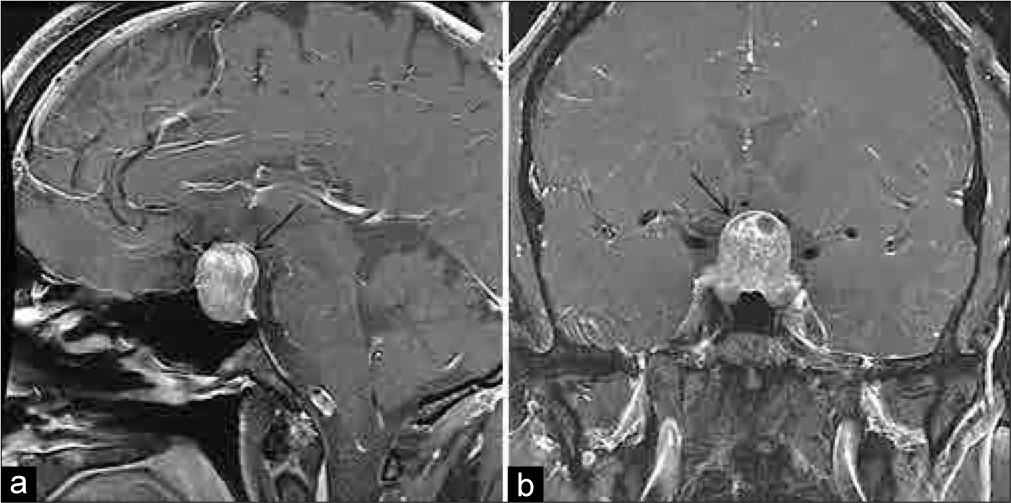
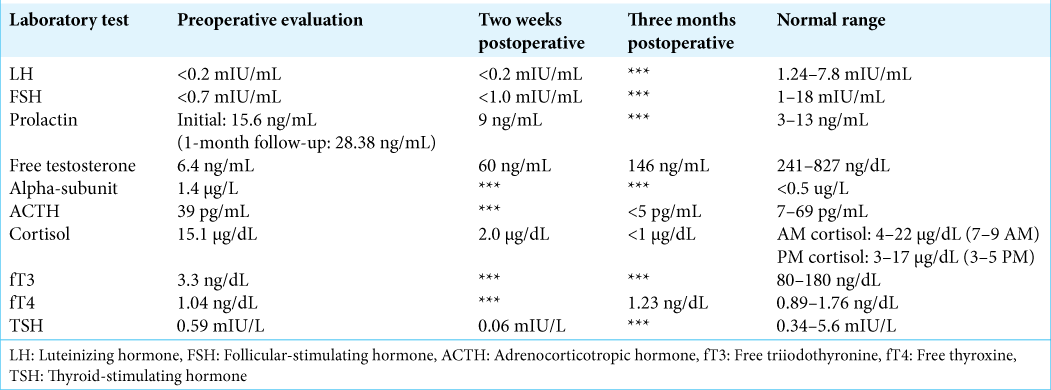
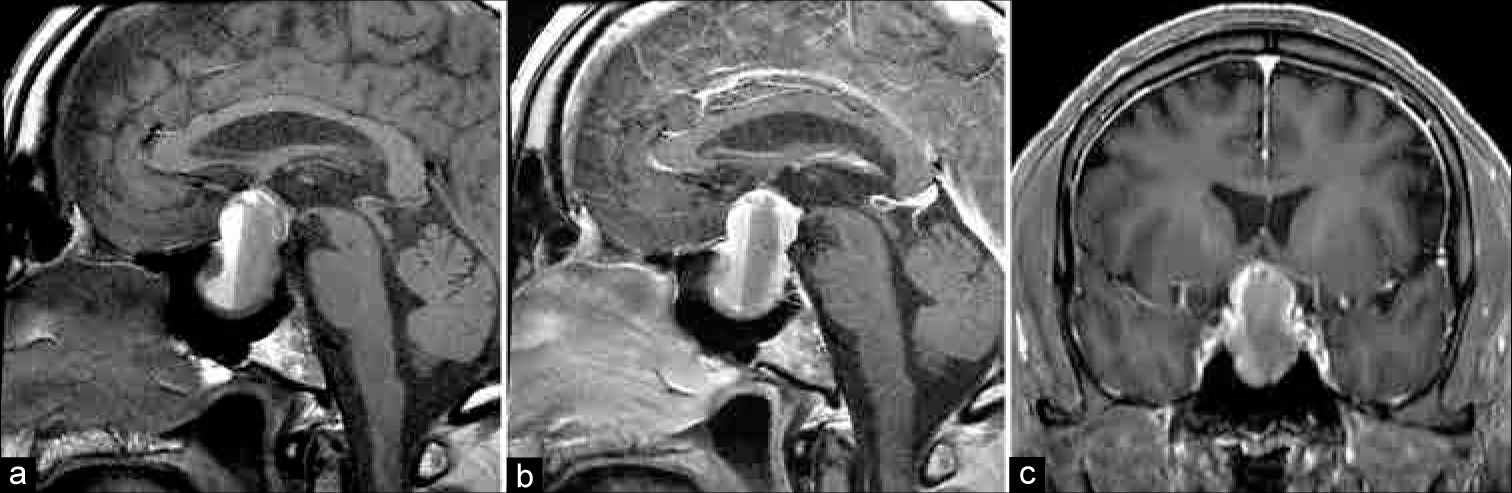
A 54-year-old male presented to his primary care physician 5 years before neurosurgical evaluation with progressive fatigue. Laboratory testing was significant for a low free testosterone of 6.4 ng/mL (normal: 247–827 ng/mL), an luteinizing hormone (LH) of <0.2 mIU/mL (normal: 1.24–7.8 mIU/mL), follicular-stimulating hormone (FSH) <0.7 mIU/mL (normal: 1–18 mIU/mL), and a borderline high prolactin of 15.6 ng/mL (normal: 3–13 ng/mL). Laboratories 1 month later were remarkable for an elevated alpha subunit of 1.4 c (normal: <0.05 μg/L), low free T3 of 3.3 ng/dL (normal: 80–180 ng/dL), and an elevated prolactin of 28.38 ng/mL (normal: 3–13 ng/mL) [
One month later, he underwent TSS. The surgery was uneventful and achieved gross total resection of the tumor and good visualization of the diaphragma sellae. The diaphragma was noted to descend into the sella turcica at the time of surgery, partially obliterating the resection cavity. The normal gland was observed layered over the diaphragma superiorly and the resection was felt to be atraumatic to the gland. There was no intraoperative evidence of a CSF leak, and no packing was placed into the sella. The sellar floor was reconstructed using a layer of Gelfoam and a horizontal bone strut harvested from the anterior sphenoid during the initial exposure. Over the first 24 h, the patient had 15 L of urine output, with an upward trending sodium from 131 mEq/L to 141 mEq/L (normal: 135–145 mEq/L). The urine output subsequently stabilized with the serum sodium remaining in the ~140 mEq/L range after serial testing. Specific gravity was 1.002 and the patient was tolerating output by matching with oral fluid intake. His urine output subsequently diminished, and he was discharged home on the 3rd postoperative day with an as-needed prescription for desmopressin (DDAVP) if increased urine output recurred. Final surgical pathology was consistent with a chromophobic pituitary adenoma.
Follow-up with his endocrinologist 2 weeks postoperatively revealed serologic evidence of panhypopituitarism, including new hypoadrenalism [
Case 2
A 58-year-old male presented to the neurosurgery clinic with a 2-year history of progressive vision loss. Initial ophthalmologic evaluation revealed bilateral cataracts, for which he underwent surgery. While he initially noted improvement, he subsequently developed unexplained right-sided vision loss. Visual field testing revealed evidence of a right-sided temporal field loss. A pituitary MRI revealed a 2.2 cm × 2.4 cm × 4.0 cm cystic sellar mass, with suprasellar extension [
Figure 3:
Case 2 pituitary MRI scan: (a) preoperative sagittal noncontrast-enhanced T1-weighted image, (b) preoperative sagittal contrast-enhanced T1-weighted image, (c) preoperative coronal noncontrast-enhanced T2-weighted image pituitary. Note the fluid-fluid level within the sella in each, consistent with subacute hemorrhage.
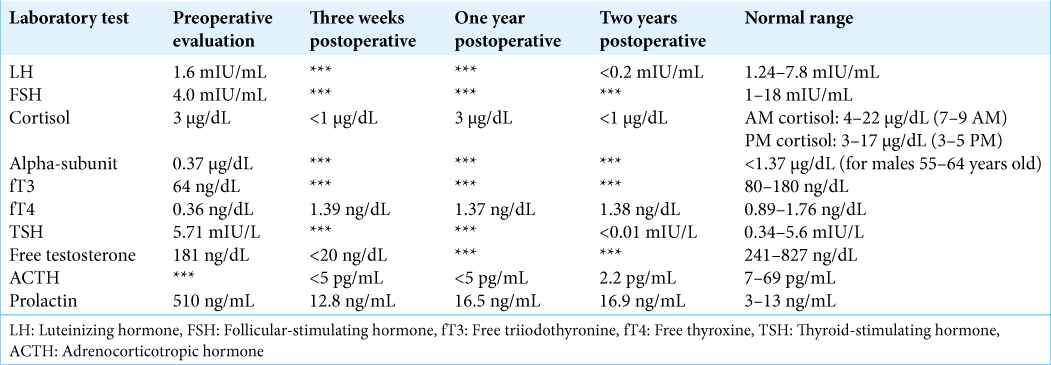
The MRI revealed a substantial cyst within the sella and suprasellar region with a fluid-fluid level consistent with subacute hemorrhage. The patient was diagnosed with a probable hemorrhagic macroprolactinoma with associated panhypopituitarism. There was no evidence of DI. Due to the sizable subacute hematoma and vision changes, TSS was recommended. Surgery was uneventful with evacuation of the hematoma and removal of a significant amount of soft tumor tissue lining the cavity. The diaphragma was noted to descend downward into the sella at the time of surgery, covered by a layer of normal appearing pituitary gland. Gross total tumor resection was felt to have been obtained with no evidence of a CSF leak. Postoperatively, he initially did well, and his vision improved, leading to discharge on postoperative day 3. The patient was discharged home on both thyroid and adrenal hormone replacement, based on his preoperative hormone levels.
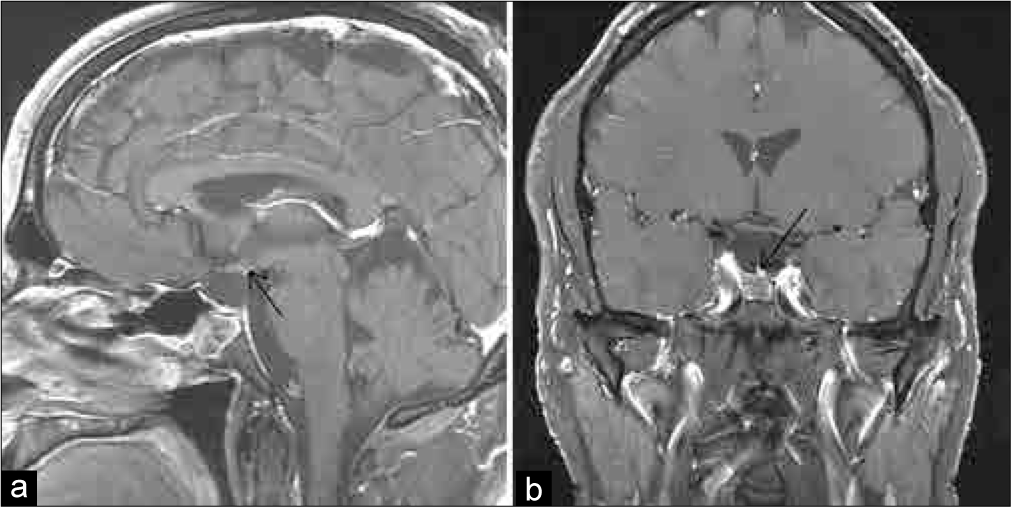
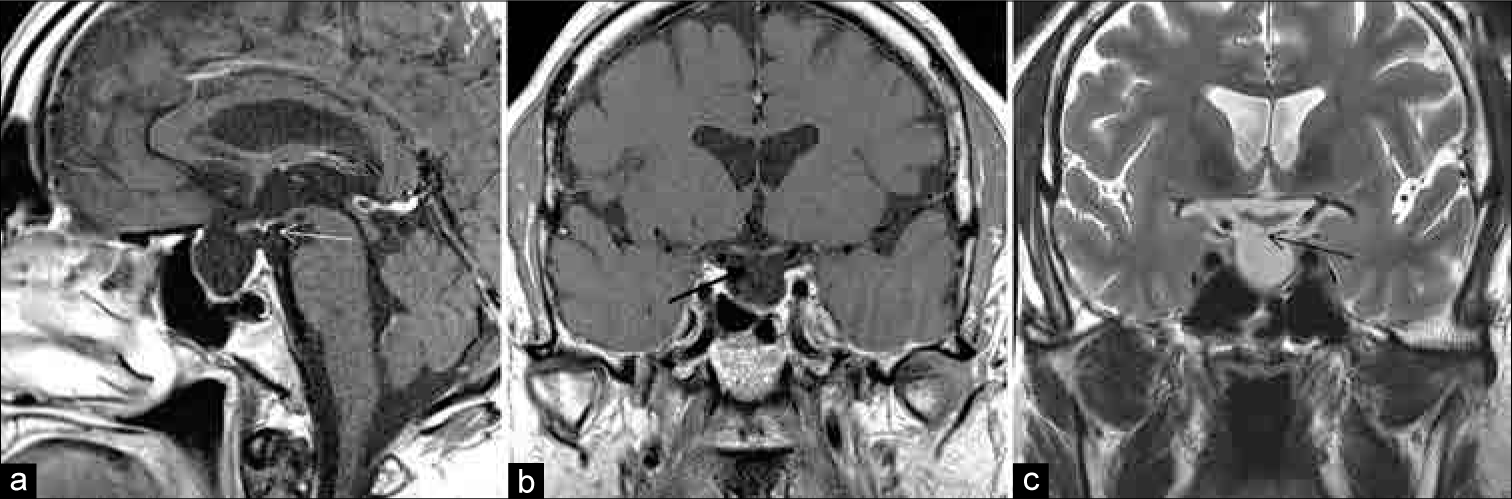
At discharge, he had no evidence of polyuria with a stable serum sodium level. He subsequently returned several days later to the emergency department with weakness, myalgia, and nausea. Laboratory testing revealed hyponatremia with a serum sodium level of 118 mEq/L (normal: 135–145 mEq/L). He was admitted to the hospital with the diagnosis of syndrome of inappropriate antidiuretic hormone secretion. This resolved over several days and he was discharged home on his previous adrenal and thyroid replacement. Over the next 4 weeks, he gradually developed symptoms consistent with DI and was started on DDAVP 5 weeks postoperatively. An MRI obtained at that time, due to the new symptoms, revealed a marked descent of the residual pituitary gland and diaphragma sellae down to the floor of the sella, with severe thinning and apparent discontinuity of the pituitary stalk [
DISCUSSION
ESS was first described in 1951 after 788 human cadavers with no history of pituitary disease were examined.[
To the best of our knowledge, the phenomenon of rapid postoperative descent of the residual pituitary gland and diaphragma into the sella with MRI evidence of pituitary stalk disruption has not been previously described. Historically, sustained postoperative pituitary endocrine dysfunction has been noted to occur in <2% of patients.[
In the two cases described in this report, the rapid descent of the residual pituitary gland into the sella turcica was felt to contribute to severe stretching of the pituitary stalk. This led to an associated increase in pituitary hormone dysfunction with the need for additional adrenal hormone replacement in one patient and the onset of delayed DI in both cases. On review of the patients’ preoperative imaging, factors that may have predisposed our two cases to this phenomenon are large tumor volume, a deep sella extending into the inferior sphenoid region and a residual normal pituitary gland centered superiorly along the diaphragma. This, therefore, allowed the descent of the entire residual pituitary gland down to the floor of the sella turcica, bringing the diaphragma and pituitary stalk down with it. A protective variable may be a gland offset to the lateral side of the sella, allowing adherence of the gland laterally and theoretically preventing this degree of descent.
As a result of this experience, we have changed the operative technique at our institution. In our current practice, we do not routinely place fat into the sella turcica during routine macroadenoma resection, unless there is evidence of an intraoperative CSF leak. However, based on these two cases and recognition of the risk factors mentioned above, we now place intrasellar fat after tumor resection to bolster the gland superiorly in those patients felt at risk. Overtime, as the fat is reabsorbed, this may promote a gentler and more gradual descent of the residual gland, allowing the pituitary stalk to elongate slowly overtime, without disruption.[
Although a rare occurrence, since adopting this practice, we have seen no further instances of this phenomenon of “pituitary ptosis,” a syndrome of postoperative ESS with MRI evidence of pituitary stalk disruption, increasing pituitary hormone dysfunction, and delayed-onset DI.
Limitations
This study is limited by its design (retrospective two-patient case series), which hinders our ability to predict the risk or severity of this phenomenon. Similarly, because routine postoperative care at our institution involves routine MRI at 3 months after surgery, we cannot comment on the exact rate of diaphragmatic descent before that time. No other cases of pituitary stalk disruption at 3 months postoperatively have been observed at our institution, but our investigation is thus at risk of bias and results may thus not be generalizable to the greater population.
Future directions
This phenomenon of postsurgical pituitary stalk disruption is important for neurosurgeons and endocrinologists to be aware of, given the potential ability to prevent subsequent postoperative pituitary endocrinopathy, a significant source of quality-of-life morbidity for pituitary adenoma patients. The rarity of symptomatic secondary ESS represents an opportunity to conduct a larger investigation to query the true prevalence of this disorder. It may be that other variables, such as obesity, may independently predict a patient’s risk. In addition, serial radiographic and laboratory examinations could better characterize the rate and degree of diaphragma descent and stalk disruption, in correspondence with panhypopituitarism. Finally, our technique of preemptive sellar packing warrants further investigation as it relates to the risk of this phenomenon.
CONCLUSION
We describe two cases of “pituitary ptosis” endocrinopathy at our institution, demonstrated by MRI evidence of pituitary stalk elongation and disruption after TSS. This is a rare syndrome of panhypopituitarism associated with DI, manifesting as progressive endocrine dysfunction in the perioperative period following macroadenoma resection. In patients at risk of this complication, placement of intrasellar fat can potentially prevent rapid descent of the gland into the sella turcica and is a rational preventative measure.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barker FG, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: Mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003. 88: 4709-19
2. Barzaghi LR, Donofrio CA, Panni P, Losa M, Mortini P. Treatment of empty sella associated with visual impairment: A systematic review of chiasmapexy techniques. Pituitary. 2018. 21: 98-106
3. Black PM, Zervas NT, Candia GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987. 20: 920-4
4. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997. 40: 225-36
5. de Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005. 90: 5471-7
6. Fouad W. Review of empty sella syndrome and its surgical management. Alexandria J Med. 2011. 47: 139-47
7. Graillon T, Passeri T, Boucekine M, Meyer M, Abritti R, Bernat AL. Chiasmapexy for secondary empty sella syndrome: Diagnostic and therapeutic considerations. Pituitary. 2021. 24: 292-301
8. Kaufman B The. “empty” sella turcica-a manifestation of the intrasellar subarachnoid space. Radiology. 1968. 90: 931-41
9. Laws ER. Autograft fat in neurological surgery. World Neurosurg. 2013. 80: 489-90
10. Maira G, Anile C, Mangiola A. Primary empty sella syndrome in a series of 142 patients. J Neurosurg. 2005. 103: 831-6
11. Olson BR, Gumowski J, Rubino D, Oldfield EH. Pathophysiology of hyponatremia after transsphenoidal pituitary surgery. J Neurosurg. 1997. 87: 499-507
12. Olson DR, Guiot G, Derome P. The symptomatic empty sella prevention and correction via the transsphenoidal approach. J Neurosurg. 1972. 37: 533-7
13. Ucciferro P, Anastasopoulou C, Empty sella.editors. Stat Pearls. US National Library of Medicine. Treasure Island, FL: Stat Pearls Publishing; 2021. p.
14. Vance ML. Perioperative management of patients undergoing pituitary surgery. Endocrinol Metab Clin North Am. 2003. 32: 355-65
15. Welch K, Stears JC. Chiasmapexy for the correction of the traction on the optic nerves and chiasm associated with their descent into an empty sella turcica. J Neurosurg. 1971. 35: 760-4