- Department of Neurosurgery, Hamilton General Hospital, McMaster University Medical Centre, Ontario, Canada,
- Faculty of Medicine and Medical Sciences, University of Balamand, El-Koura, Lebanon.
Correspondence Address:
Mohamed Rashed Alhantoobi, Department of Neurosurgery, Hamilton General Hospital, McMaster University Medical Centre, Ontario, Canada.
DOI:10.25259/SNI_125_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohamed Rashed Alhantoobi1, Mohamad Ali Kesserwan1, Hassan A. Khayat1, Mohammad Lawasi2, Sunjay Sharma1. Rates of cerebrospinal fluid leak and pseudomeningocele formation after posterior fossa craniotomy versus craniectomy: A systematic review and meta-analysis. 21-Apr-2023;14:140
How to cite this URL: Mohamed Rashed Alhantoobi1, Mohamad Ali Kesserwan1, Hassan A. Khayat1, Mohammad Lawasi2, Sunjay Sharma1. Rates of cerebrospinal fluid leak and pseudomeningocele formation after posterior fossa craniotomy versus craniectomy: A systematic review and meta-analysis. 21-Apr-2023;14:140. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12283
Abstract
Background: Postoperative cerebrospinal fluid (CSF) leak is a major concern after posterior fossa surgery with significant clinical implications. It has been postulated that replacing the bone flap, performing a craniotomy, would reinforce the surgical closure and decrease rates of CSF leak. This systematic review and meta-analysis compared the rate of CSF leak after posterior fossa craniotomies versus craniectomies.
Methods: Three databases were searched for English studies comparing the primary outcome, rate of CSF leak, after adult posterior fossa craniotomies versus craniectomies. Secondary outcomes included the rate of postoperative pseudomeningocele formation, CSF leak and pseudomeningocele formation, CSF diversion, revision surgery, and infection. Pooled estimates and relative risks for dichotomous outcomes were calculated using Review Manager 5.4, with corresponding 95% confidence intervals (CIs).
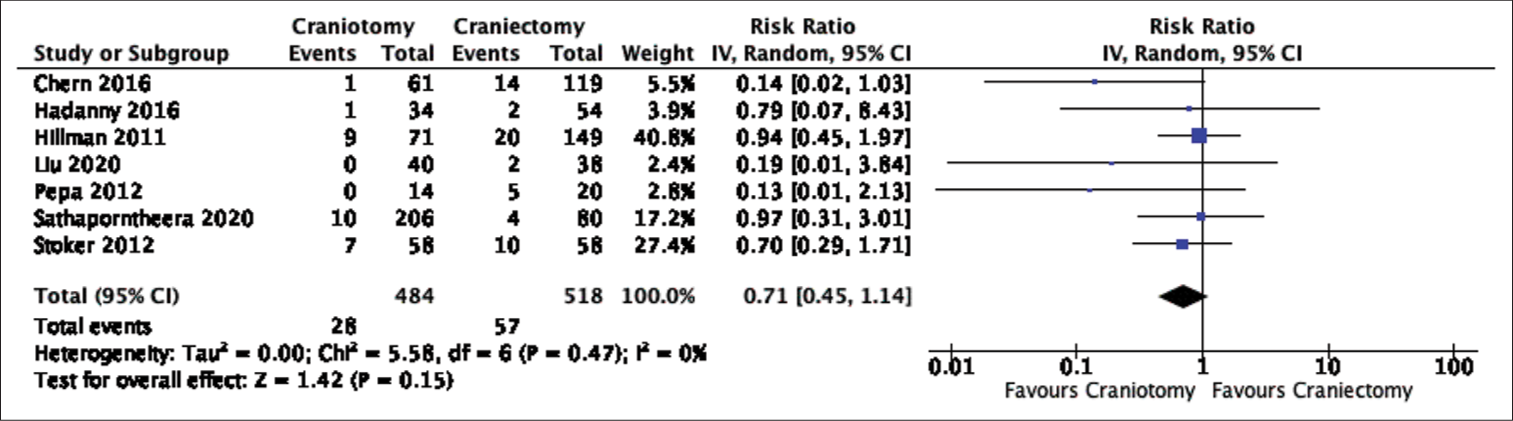
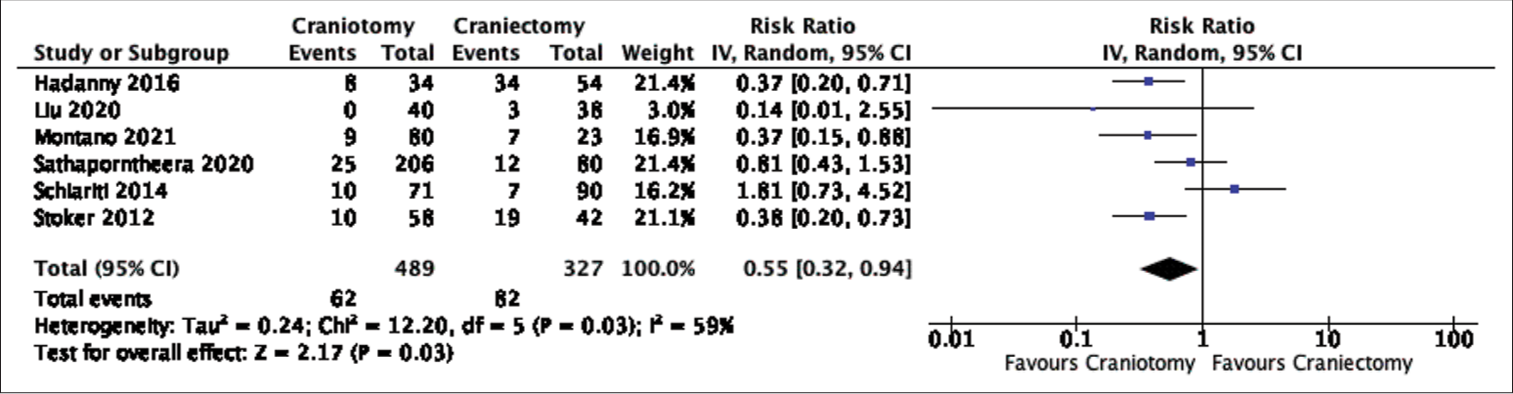
Results: A total of 1250 patients (635 craniotomies and 615 craniectomies), from nine studies, were included in the final analysis. Even though rates of CSF leak favored craniotomies, the difference did not reach statistical significance in our pooled analysis (Risk Ratio: 0.71, 95% Confidence Interval: 0.45-1.14, p-value = 0.15, Heterogeneity I-squared = 0%). On the other hand, comparing the rates of pseudomeningocele formation and CSF leak, as a combined outcome, or pseudomeningocele formation only showed a significant difference favoring craniotomies. The quality of evidence in this meta-analysis was graded as having a high risk of bias based on the risk of bias in non-randomized studies - of exposure criteria.
Conclusion: Based on evidence with high risk of bias, rates of postoperative CSF leak and pseudomeningocele formation favored posterior fossa craniotomies over craniectomies. Further research with more robust methodology is required to validate these findings.
Keywords: Cerebrospinal fluid leak, Craniectomy, Cranioplasty, Craniotomy, Posterior cranial fossa, Pseudomeningocele
INTRODUCTION
Posterior fossa surgeries are common and essential in neurosurgery, as they treat a variety of pathologies in a compact space filled with vital anatomy.[
MATERIALS AND METHODS
Search strategy and study selection
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis and the Cochrane Handbook for Systematic Reviews of Interventions.[
Data collection
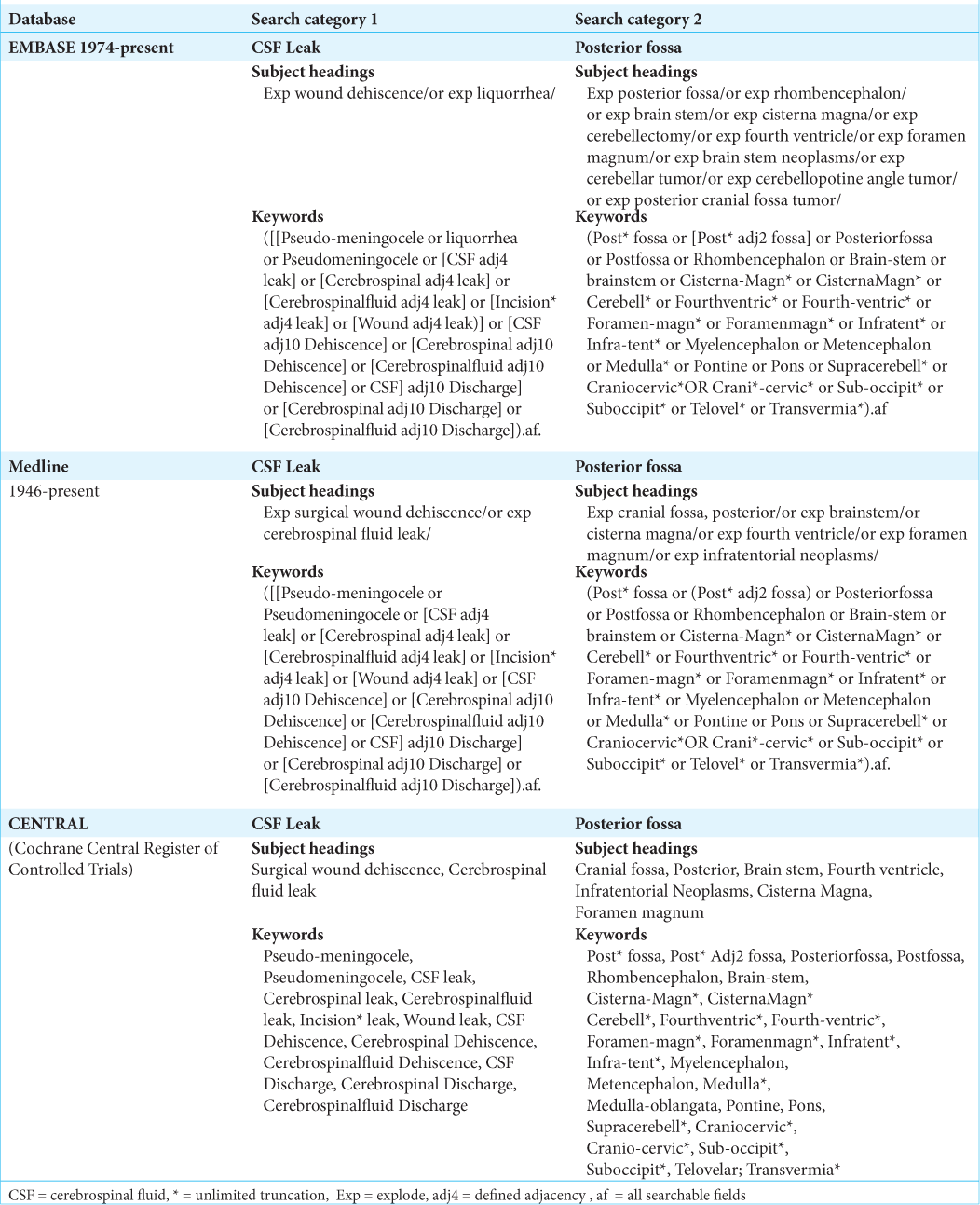
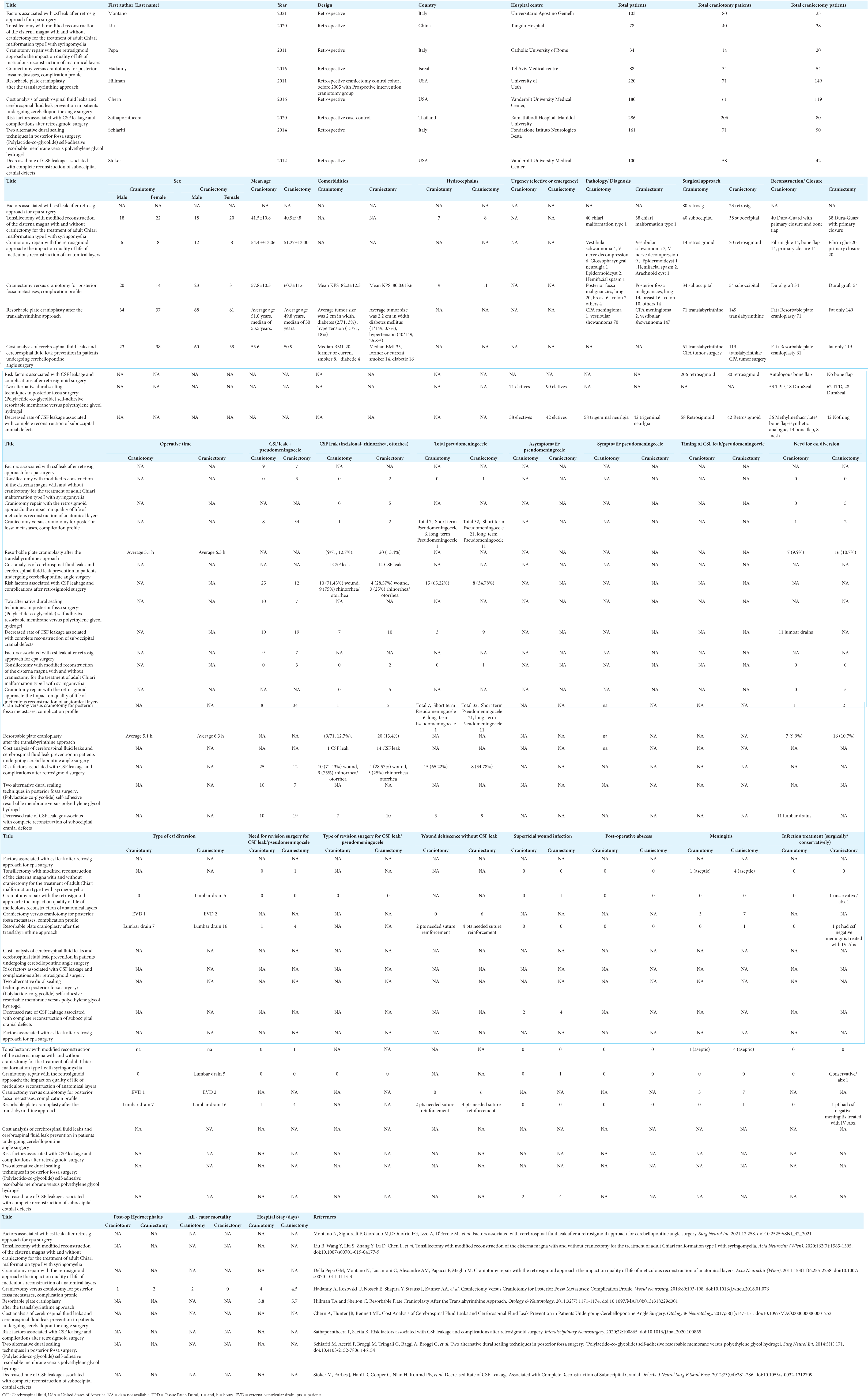
Data regarding study characteristics, patient demographics, surgical indications and approaches, closure techniques, postoperative complications, postoperative hospital stay, and all-cause mortality were collected. The primary outcome investigated was the rate of postoperative CSF leak. CSF leak included incisional CSF leak, rhinorrhea, or otorrhea. Secondary outcomes included postoperative rate of CSF leak and pseudomeningocele formation, pseudomeningocele formation only, CSF diversion, revision surgery, superficial wound infection, intracranial abscess, and meningitis. Pseudomeningoceles were included if clinically or radiologically diagnosed. The complete data abstraction table is provided in Supplementary Table 2.
Data analysis
Rates of CSF leak and related complications after posterior fossa craniotomy versus craniectomy surgeries were pooled using Review Manager (version 5.4.1, Cochrane Collaboration) by the inverse variance method and random effects analysis model. The pooled results of the eight dichotomous outcomes were presented as risk ratio (RR) with a corresponding 95% confidence interval (CI). The statistical significance was defined as P < 0.05. Quality of evidence was assessed using the Risk of Bias In Non-randomized Studies - of Exposure (ROBINS-E).[
RESULTS
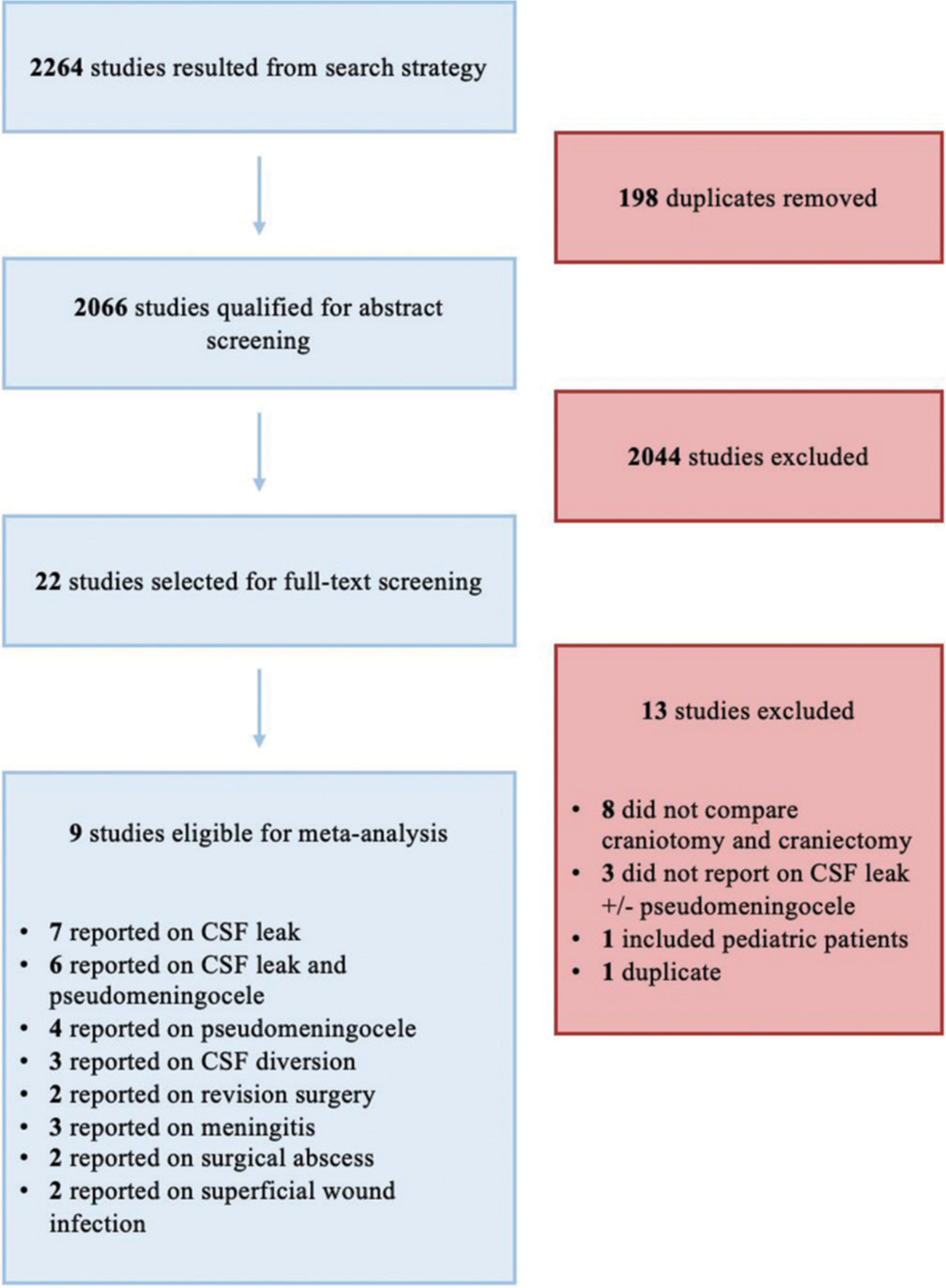
This systematic review identified 2264 studies, with 198 of those representing duplicate publications. Of the remaining articles, 2044 studies were excluded after title and abstract screening. Full-text review of the resulting 22 articles excluded another 13 studies. The remaining nine full-text retrospective studies[
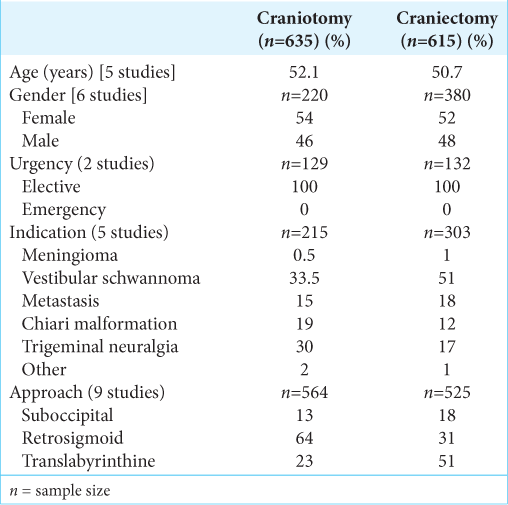
In the craniotomy group, the average age based on five studies was 52.1 years. Six studies reported patient gender of which 54% were female. Five studies detailed the indication for surgery: vestibular schwannoma 33.5%, trigeminal neuralgia 30%, chiari malformation 19%, metastasis 15%, meningioma 0.5%, and other 2%. Eight articles mentioned the surgical procedure: retrosigmoid 64%, translabrynthine 23%, and suboccipital 13% craniotomies. Only two papers documented on surgical urgency; all surgeries were performed on elective bases [
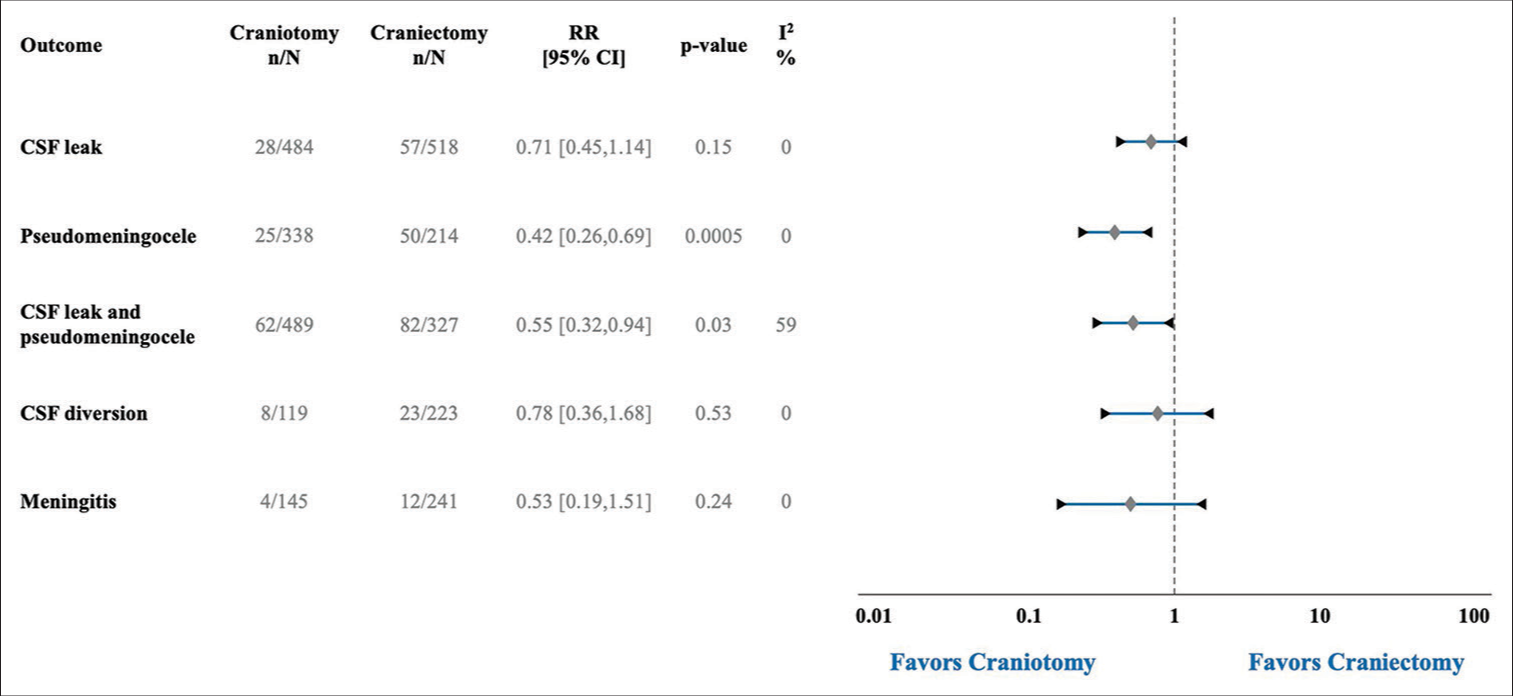
Seven studies reported on CSF leak with a total of 85 events over 1002 cases. CSF leak occurred after 28 of 484 craniotomies (5.79%) and 57 of 518 craniectomies (11.00%). Even though CSF leak was almost twice as common after craniectomies, there was no statistical difference in the pooled analysis (Risk Ratio (RR): 0.71, 95% Confidence Interval (CI) 0.45-1.14, p-value (P) = 0.15, Heterogeneity I-squared (I^2) = 0%) [
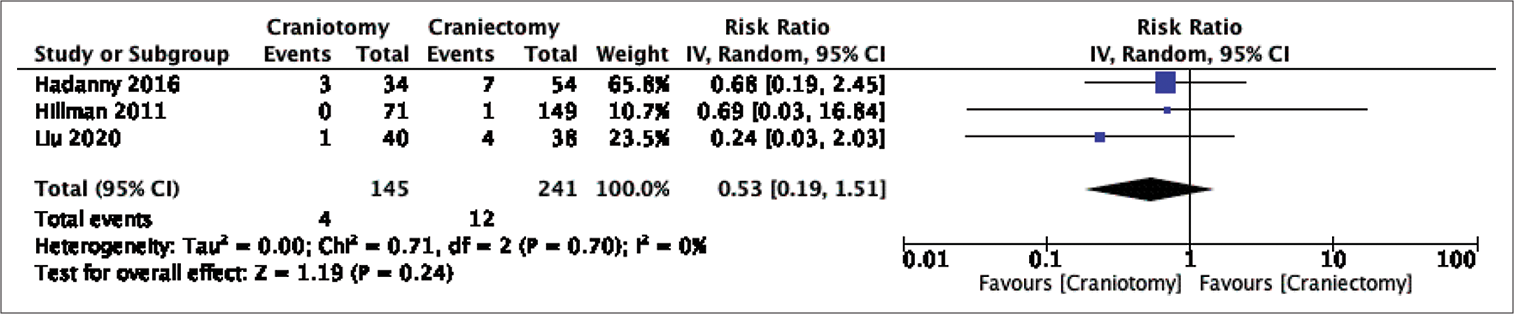
The need for postoperative CSF diversion was recorded in three articles with a total of 31 diversions after 342 surgeries. CSF diversion was required after 8 of 119 craniotomies (6.72%) and 23 of 223 craniectomies (10.31%). There was no significant difference between the two groups in the pooled analysis (RR: 0.78, 95 % CI 0.36–0.1.68, P = 0.53, I2 = 0%) [
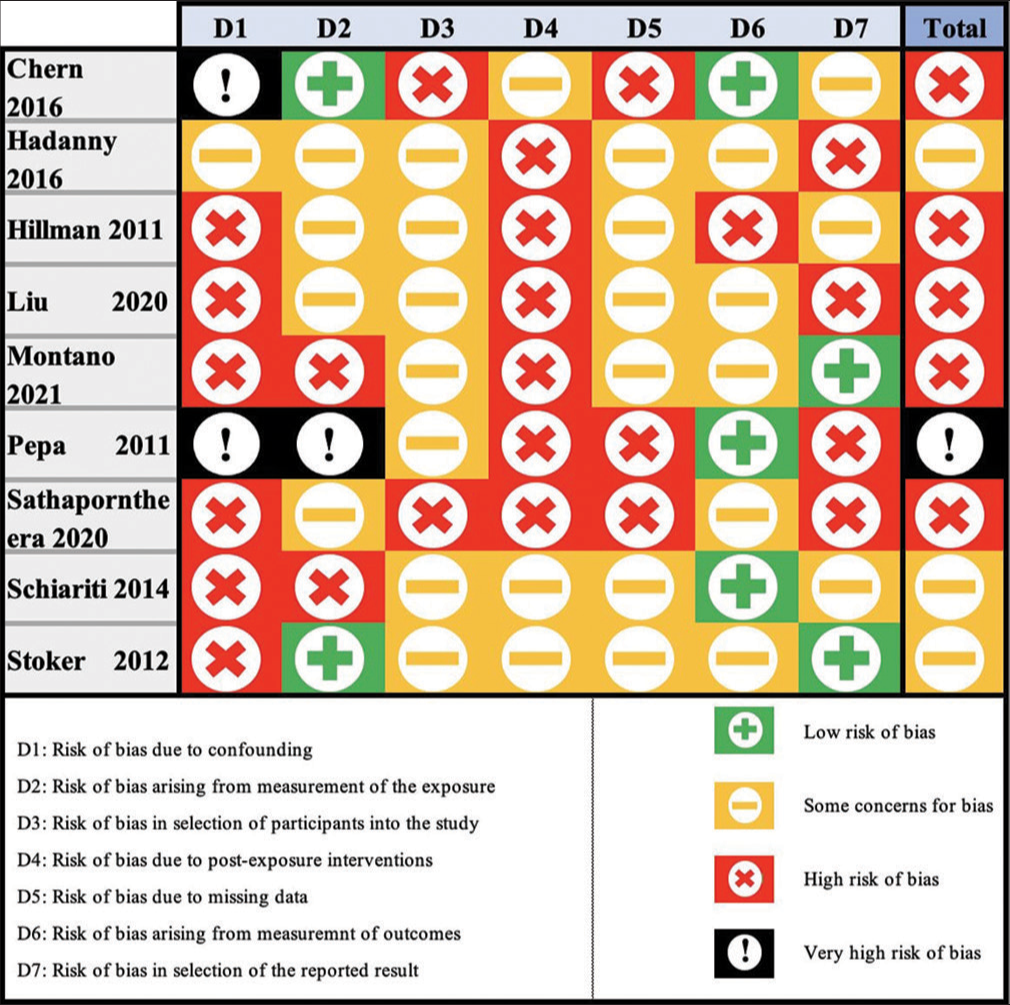
The quality of evidence in this meta-analysis was graded using the ROBINS-E criteria. Three studies were graded as having some concerns for bias, five were graded as having a high risk of bias, and one had a very high risk of bias [
DISCUSSION
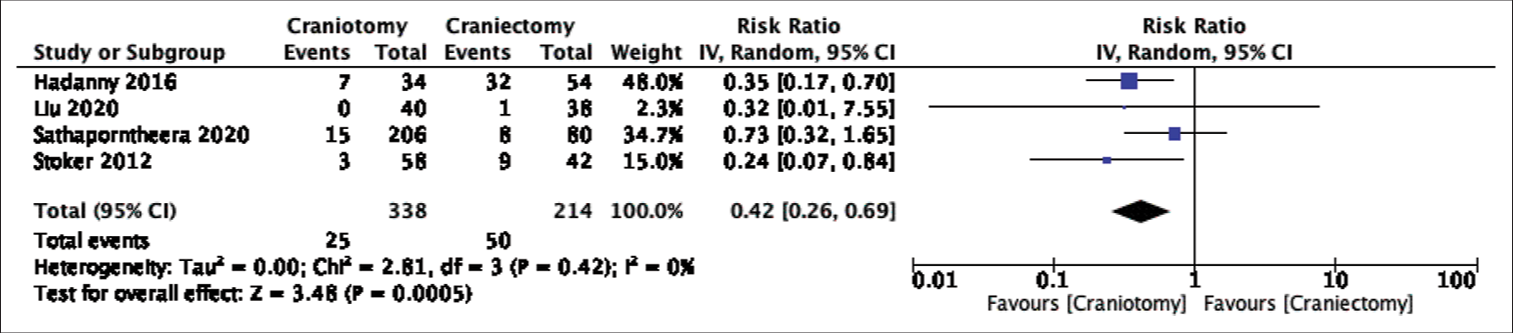
In this systematic review and meta-analysis, data were pooled from nine retrospective studies comparing the rates of CSF leak or pseudomeningocele formation after posterior fossa craniotomies versus craniectomies. In terms of our primary outcome, the postoperative rate of CSF leak did not differ significantly between the two groups despite the craniectomy group having almost double the incidence of CSF leak (5.79% vs. 11.00%). On the other hand, pooled data demonstrated a statistically lower incidence of postoperative CSF leak and pseudomeningocele formation, as a combined outcome, or pseudomeningocele formation alone after craniotomy surgeries. The difference in significance between the rates of CSF leak and combined outcome, CSF leak and pseudomeningocele formation, is likely attributed to the larger proportion of cases with postoperative pseudomeningocele formation (13.59%) than CSF leak (8.48%). This could potentially skew the result towards favoring craniotomies given their significantly lower occurrence of pseudomeningocele formation. In addition, only two studies were included in the combined outcome but not in the CSF leak comparison;[
Unfortunately, there was a lack of sufficient data to adequately compare complications related to CSF leak and pseudomeningocele formation such as postoperative infections and interventions. Only the postoperative rate of meningitis (16 events) and need of CSF diversion (31 events) were included in the meta-analysis with no significant difference between the surgical groups in either outcome. Further prospective studies are required to further investigate the occurrence and impact of CSF leak and pseudomeningocele formation after posterior fossa surgeries.
There are multiple limitations to this systematic review and meta-analysis. The first limitation is pertaining to the low quality of evidence with high risk of bias based on the ROBINS-E criteria. This is heavily influenced by the retrospective nature of included studies. As previously mentioned, paucity of data also precluded extensive and comprehensive analyses on the impact of craniotomies on posterior fossa CSF leak, pseudomeningocele formation, and related complications. Furthermore, various confounding factors and heterogeneity between studies may have influenced the outcomes of this analysis; factors such as patient demographics, pathology, surgical procedure, closure technique, and other concurrent therapies such as steroids and radiation.[
CONCLUSION
This systematic review and meta-analysis is the first to compare the postoperative rate of CSF leak and pseudomeningocele formation after posterior fossa craniotomies versus craniectomies. The results of this study favored craniotomies over craniectomies in terms of CSF leak (P = 0.15, non-significant) and pseudomeningocele formation (P < 0.05, significant). Given these findings, and in the absence of evidence implicating posterior fossa craniotomies with higher surgical risks, neurosurgeons should consider performing craniotomies for posterior fossa surgeries. Further prospective studies are required to determine and validate the implications of posterior fossa craniotomy in reducing the risk of postoperative CSF leak and pseudomeningocele formation.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY TABLES
SUPPLEMENTARY FIGURES
Supplementary Figure 1.2:
Forest plot of cerebrospinal fluid leak and pseudomeningocele formation comparison. [IV = inverse variance, CI = confidence interval, Tau^2 = Tau-square between-study variance, Chi^2 = Chi-square statistic, df = degrees of freedom, P = p-value, I^2 = heterogeneity I-squared, Z = Z-test].
References
1. Altaf I, Vohra AH, Shams S. Management of cerebrospinal fluid leak following posterior cranial fossa surgery. Pak J Med Sci. 2016. 32: 1439-43
2. Becker SS, Jackler RK, Pitts LH. Cerebrospinal fluid leak after acoustic neuroma surgery: A comparison of the translabyrinthine, middle fossa, and retrosigmoid approaches. Otol Neurotol. 2003. 24: 107-12
3. Beer-Furlan A, Vellutini EA, Gomes MQ, Cardoso AC, Prevedello LM, Todeschini AB. Approach selection and surgical planning in posterior cranial fossa Meningiomas: How I do it. J Neurol Surg B Skull Base. 2019. 80: 380-91
4. Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J. The risk of bias in observational studies of exposures (ROBINS-E) tool: Concerns arising from application to observational studies of exposures. Syst Rev. 2018. 7: 242
5. Brennan JW, Rowed DW, Nedzelski JM, Chen JM. Cerebrospinal fluid leak after acoustic neuroma surgery: Influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001. 94: 217-23
6. Chern A, Hunter JB, Bennett ML. Cost analysis of cerebrospinal fluid leaks and cerebrospinal fluid leak prevention in patients undergoing cerebellopontine angle surgery. Otol Neurotol. 2017. 38: 147-51
7. della Pepa GM, Montano N, Lucantoni C, Alexandre AM, Papacci F, Meglio M. Craniotomy repair with the retrosigmoid approach: The impact on quality of life of meticulous reconstruction of anatomical layers. Acta Neurochir (Wien). 2011. 153: 2255-8
8. Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D. Complications of posterior cranial fossa surgery-an institutional experience of 500 patients. Surg Neurol. 2009. 72: 369-75
9. Grotenhuis JA. Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol. 2005. 64: 490-3
10. Hadanny A, Rozovski U, Nossek E, Shapira Y, Strauss I, Kanner AA. Craniectomy versus craniotomy for posterior fossa metastases: Complication profile. World Neurosurg. 2016. 89: 193-8
11. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3. London: Cochrane; 2022. p.
12. Hillman TA, Shelton C. Resorbable plate cranioplasty after the translabyrinthine approach. Otol Neurotol. 2011. 32: 1171-4
13. Kehler U, Hirdes C, Weber C, Spuck S, Tronnier V, Kundt G. CSF leaks after cranial surgery-a prospective multicenter analysis. Innov Neurosurg. 2013. 1: 49-53
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009. 62: e1-34
15. Liu B, Wang Y, Liu S, Zhang Y, Lu D, Chen L. Tonsillectomy with modified reconstruction of the cisterna magna with and without craniectomy for the treatment of adult Chiari malformation Type I with syringomyelia. Acta Neurochir (Wien). 2020. 162: 1585-95
16. McMullin JL, Hu QL, Merkow RP, Bilimoria KY, Hu YY, Ko CY. Are kids more than just little adults? A comparison of surgical outcomes. J Surg Res. 2022. 279: 586-91
17. Montano N, Signorelli F, Giordano M, D’Onofrio FG, Izzo A, D’Ercole M. Factors associated with cerebrospinal fluid leak after a retrosigmoid approach for cerebellopontine angle surgery. Surg Neurol Int. 2021. 12: 258
18. Rodgers GK, Luxford WM. Factors affecting the development of cerebrospinal fluid leak and meningitis after translabyrinthine acoustic tumor surgery. Laryngoscope. 1993. 103: 959-62
19. Sastry RA, Walek K, Leary OP, Rex N, Shaaya EA, Poggi JA. Incidence, characteristics, and outcomes of pseudomeningocele and cerebrospinal fluid fistula after posterior fossa surgery. World Neurosurg. 2022. 164: e1094-102
20. Sathaporntheera P, Saetia K. Risk factors associated with CSF leakage and complications after retrosigmoid surgery. Interdiscip Neurosurg. 2020. 22: 100865
21. Schiariti M, Acerbi F, Broggi M, Tringali G, Raggi A, Broggi G. Two alternative dural sealing techniques in posterior fossa surgery: (Polylactide-co-glycolide) self-adhesive resorbable membrane versus polyethylene glycol hydrogel. Surg Neurol Int. 2014. 5: 171
22. Stoker MA, Forbes JA, Hanif R, Cooper C, Nian H, Konrad PE. Decreased rate of CSF leakage associated with complete reconstruction of Suboccipital cranial defects. J Neurol Surg B Skull Base. 2012. 73: 281-6
23. Udani V, Holly LT, Chow D, Batzdorf U. Posterior fossa reconstruction using titanium plate for the treatment of cerebellar ptosis after decompression for Chiari malformation. World Neurosurg. 2014. 81: 836-41