- Department of Pathology, University of Miami Miller School of Medicine, Miami, Florida, United States.
- Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, Florida, United States.
Correspondence Address:
Khadeja Khan, Department of Pathology University of Miami Miller School of Medicine, Miami, Florida, United States.
DOI:10.25259/SNI_423_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Khadeja Khan, Evan Luther, Alexis A. Morrell, Sze Kiat Tan, Daniel G. Eichberg, Ashish H. Shah, Victor M. Lu, Sakir H. Gultekin, Jacques J. Morcos. Recurrent adult pilocytic astrocytoma presenting with intraventricular and leptomeningeal spread. 19-Jul-2021;12:359
How to cite this URL: Khadeja Khan, Evan Luther, Alexis A. Morrell, Sze Kiat Tan, Daniel G. Eichberg, Ashish H. Shah, Victor M. Lu, Sakir H. Gultekin, Jacques J. Morcos. Recurrent adult pilocytic astrocytoma presenting with intraventricular and leptomeningeal spread. 19-Jul-2021;12:359. Available from: https://surgicalneurologyint.com/surgicalint-articles/10979/
Abstract
Background: Infratentorial pilocytic astrocytomas are uncommon tumors in adulthood but are thought to be prognostically similar to their pediatric counterparts with excellent overall survival following gross total resection. However, given the relative rarity of these tumors, no management guidelines exist for recurrent adult pilocytic astrocytomas (APAs). This lack of consensus is especially problematic for inoperable recurrences or those with aggressive features concerning for malignant transformation.
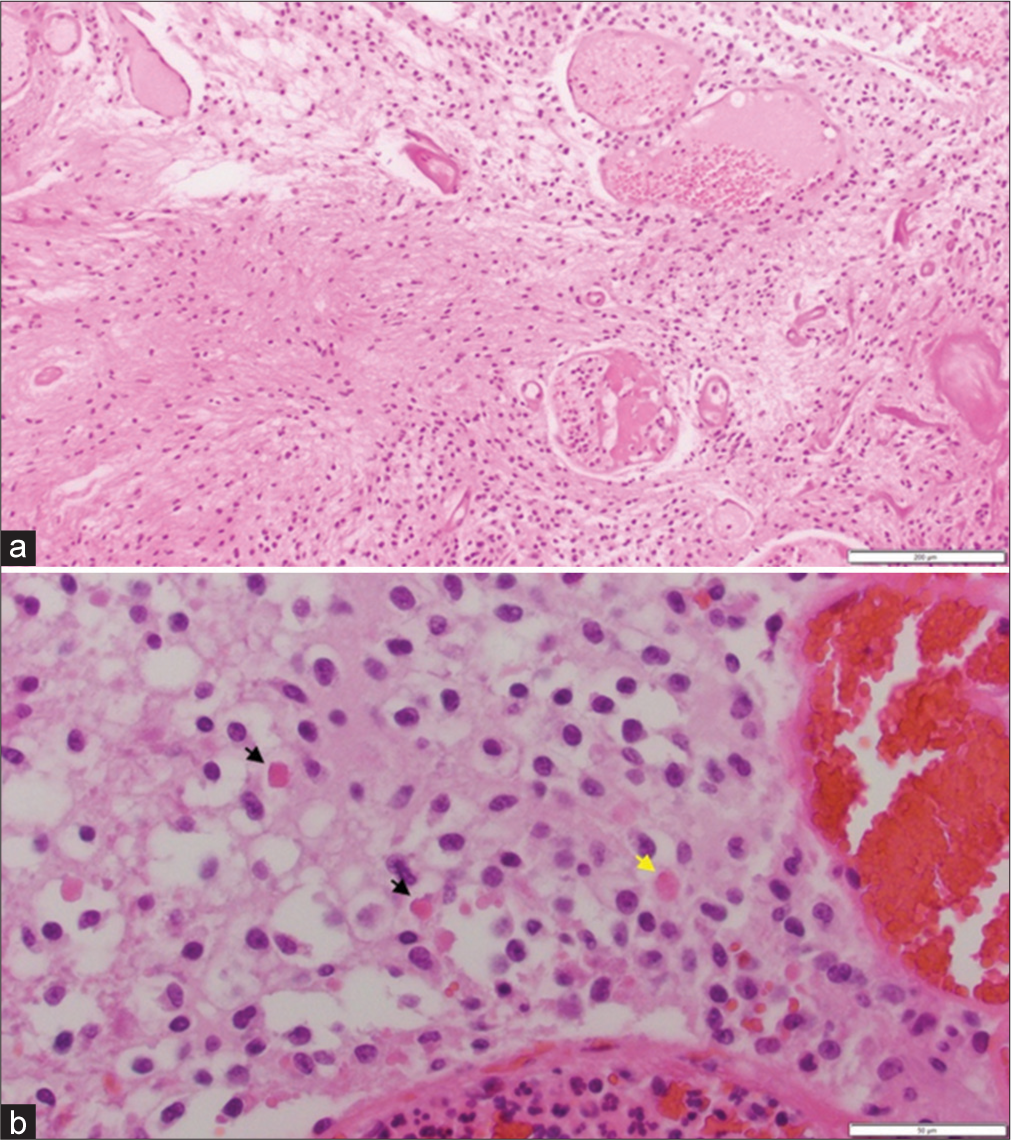
Case Description: In 2017, a 26-year-old female presented with headaches, nausea, vomiting, and blurry vision. A brain magnetic resonance imaging (MRI) demonstrated a large, well-circumscribed mass within the fourth ventricle causing obstructive hydrocephalus. She underwent near-total resection through a midline suboccipital transtonsillar approach. Pathology demonstrated a World Health Organization Grade 1 pilocytic astrocytoma. Despite initial improvement in her symptoms, she developed worsening headaches and lethargy 10 months after surgery and repeat MRI demonstrated recurrent tumor within the entire ventricular system and the subarachnoid spaces of the left cerebellopontine angle suggesting leptomeningeal spread. Due to the unresectable nature of the recurrence, the patient declined any further intervention and succumbed to her disease 6 months later.
Conclusion: We present the first case of a recurrent APA presenting with intraventricular and leptomeningeal spread. Although thought to be a benign neoplasm, close interval follow-up with serial imaging is of essential, especially in those patients with known residual tumor, to prevent aggressive recurrences such as this.
Keywords: Adult pilocytic astrocytoma, Inoperable recurrence, Leptomeningeal spread, Malignant transformation, Subtotal resection
INTRODUCTION
Pilocytic astrocytomas are one of the most common benign brain neoplasms of childhood but remain exceedingly rare in adults accounting for only 0.8% of central nervous system tumors in patients >20 years of age.[
CLINICAL PRESENTATION
An otherwise healthy 26-year-old female presented in 2017 with severe headaches, nausea, vomiting, and visual changes. After fundoscopic examination demonstrated Grade 5 papilledema, contrast-enhanced brain magnetic resonance imaging (MRI) was performed revealing a 4.8 × 4.4 × 4.1-cm mass centered in the fourth ventricle resulting in obstructive hydrocephalus [
Figure 1:
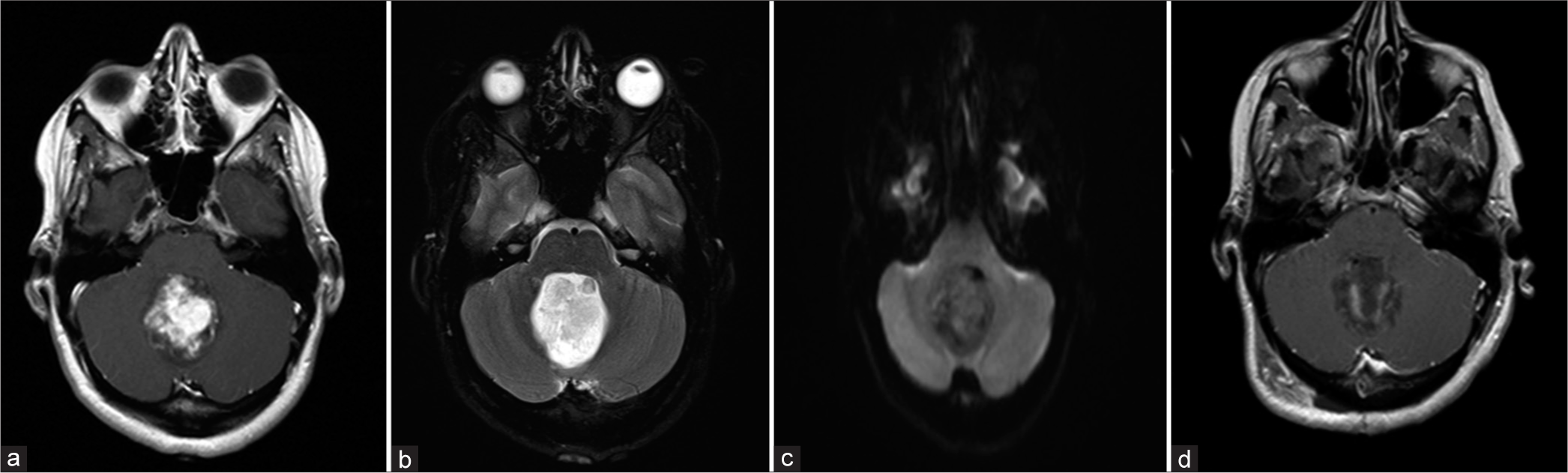
Imaging performed in April 2017. (a) Preoperative T1 contrast enhancing MRI demonstrating a well-circumscribed lesion within the fourth ventricle. (b) Preoperative T2 MRI demonstrating no significant surrounding edema or infiltration of the normal brain parenchyma. (c) Preoperative DWI demonstrating no diffusion restriction. (d) Postoperative T1 contrast enhancing MRI demonstrating no obvious residual tumor.
Figure 3:
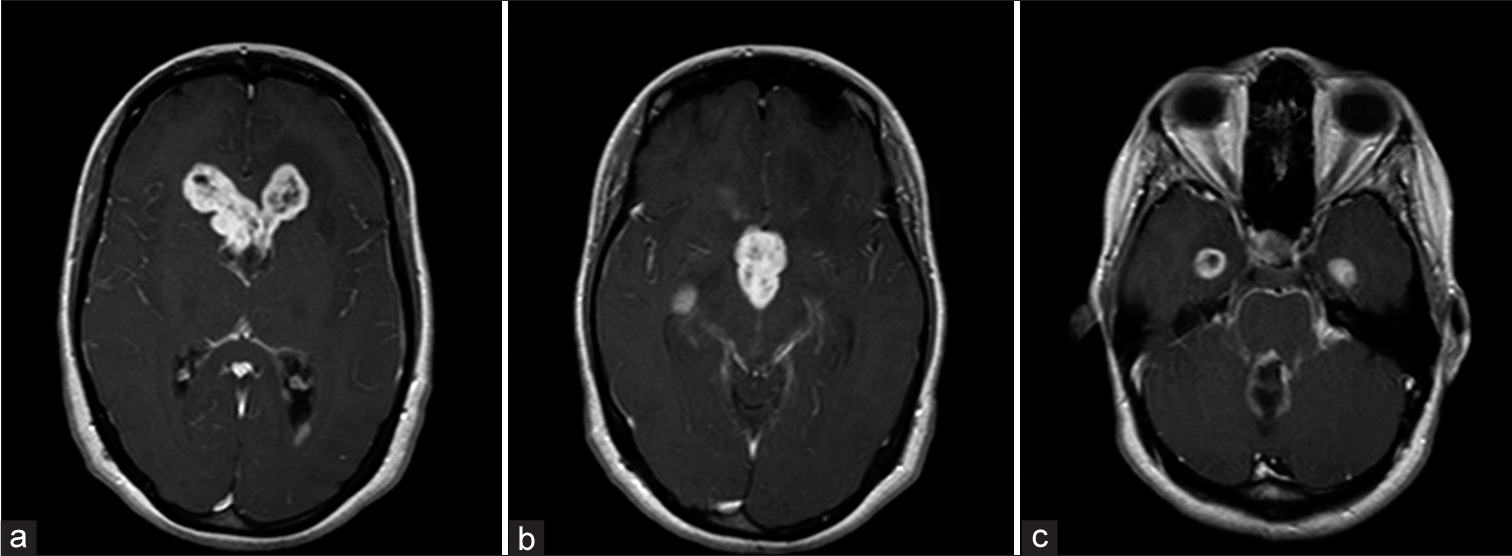
Imaging performed in March 2018. (a) T1 contrast-enhanced MRI demonstrating tumor recurrence within the frontal horns of the lateral ventricle. (b) T1 MRI demonstrating recurrent tumor within the third ventricle. (c) T1 MRI demonstrating leptomeningeal enhancement in the left cerebellopontine angle and temporal horns.
DISCUSSION
Pilocytic astrocytomas are one of the most common gliomas of childhood. However, in adults, they are much less frequent with an incidence of 3.4/1 million.[
Several studies have shown that increasing patient age is correlated with lower rates of overall survival in pilocytic astrocytomas with 5-year survival dropping from 96.5% in pediatric patients to approximately 53% in patients older than 60.[
In the pediatric population, several retrospective series examined the benefit of surveillance imaging for pilocytic astrocytomas and concluded that patients with a gross total resection have a very low likelihood of recurrence and may not benefit from long-term surveillance.[
Little data exist to help guide postsurgical adjuvant therapy for subtotally resected APAs. Radiation therapy (XRT) has been evaluated in several studies with mixed findings. A recent study evaluating overall survival following subtotal resection of APAs found that those patients who received postoperative XRT had significantly higher mortality rates compared to those that received surveillance alone.[
Although no standard chemotherapeutic regimen exists for APAs, various drug combinations, with or without XRT, have been used in recurrent/inoperable pilocytic astrocytomas with varying degrees of effectiveness.[
Leptomeningeal disease in recurrent APAs remains significantly difficult to manage because, by definition, it is unresectable. Biopsy may be warranted to determine if the lesion has malignantly transformed. In addition, molecular testing may also increase diagnostic and prognostic accuracy as DNA methylation has been shown to help determine tumor grade when histologic features alone remain ambiguous.[
In our patient, the tumor involved the fourth ventricle and resection may have led to leptomeningeal spread within the CPA. However, this does not fully explain how the tumor casted the entire ventricular system. Given that the patient was shunted for hydrocephalus, this may have led to ventricular seeding yet this would likely have tracked along the catheter rather than involving even the contralateral lateral ventricle.[
CONCLUSION
We report a rare case of a recurrent APA presenting with diffuse intraventricular and leptomeningeal spread. This atypical and fatal case highlights the need for improved management guidelines and better diagnostic criteria for these tumors in the adult population. Novel molecular profiling may help detect subtle differences in tumor grade and, in turn, better direct adjuvant therapy in patients with known residual or recurrent disease. Although thought to be a benign neoplasm, close interval follow-up with serial imaging may be useful in those patients with known residual tumor.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alford R, Gargan L, Bowers DC, Klesse LJ, Weprin B, Koral K. Postoperative surveillance of pediatric cerebellar pilocytic astrocytoma. J Neurooncol. 2016. 130: 149-54
2. Bian SX, McAleer MF, Vats TS, Mahajan A, Grosshans DR. Pilocytic astrocytoma with leptomeningeal dissemination. Childs Nerv Syst. 2013. 29: 441-50
3. Bond KM, Hughes JD, Porter AL, Orina J, Fang S, Parney IF. Adult pilocytic astrocytoma: An institutional series and systematic literature review for extent of resection and recurrence. World Neurosurg. 2018. 110: 276-83
4. Bowers DC, Krause TP, Aronson LJ, Barzi A, Burger PC, Carson BS. Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg. 2001. 34: 229-34
5. Brown MT, Friedman HS, Oakes WJ, Boyko OB, Hockenberger B, Schold SC. Chemotherapy for pilocytic astrocytomas. Cancer. 1993. 71: 3165-72
6. Brown PD, Buckner JC, O’Fallon JR, Iturria NL, Brown CA, O’Neill BP. Adult patients with supratentorial pilocytic astrocytomas: A prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2004. 58: 1153-60
7. Burkhard C, Di Patre PL, Schuler D, Schuler G, Yasargil MG, Yonekawa Y. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003. 98: 1170-4
8. Coelho J, Nunes S, Salgado D. Spontaneous malignant transformation of a pilocytic astrocytoma of cerebellum: Case report. Child Neurol Open. 2015. 2: 1-5
9. Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015. 129: 775-88
10. Dodgshun AJ, Maixner WJ, Hansford JR, Sullivan MJ. Low rates of recurrence and slow progression of pediatric pilocytic astrocytoma after gross-total resection: Justification for reducing surveillance imaging. J Neurosurg Pediatr. 2016. 17: 569-72
11. Dorward IG, Luo J, Perry A, Gutmann DH, Mansur DB, Rubin JB. Postoperative imaging surveillance in pediatric pilocytic astrocytomas. J Neurosurg Pediatr. 2010. 6: 346-52
12. Ellis JA, Waziri A, Balmaceda C, Canoll P, Bruce JN, Sisti MB. Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J Neurooncol. 2009. 95: 377-82
13. Green R, Woyshner E, Quan J, Pope W, Cloughesy T. Treatment of unresectable adult pilocytic astrocytoma with bevacizumab with or without temozolomide (P01.097). Neurology. 2013. 80: P01097
14. Hallemeier CL, Pollock BE, Schomberg PJ, Link MJ, Brown PD, Stafford SL. Stereotactic radiosurgery for recurrent or unresectable pilocytic astrocytoma. Int J Radiat Oncol Biol Phys. 2012. 83: 107-12
15. Ishkanian A, Laperriere NJ, Xu W, Millar BA, Payne D, Mason W. Upfront observation versus radiation for adult pilocytic astrocytoma. Cancer. 2011. 117: 4070-9
16. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: Analysis of the surveillance, epidemiology, and end results program of the national cancer institute. J Neurooncol. 2012. 108: 187-93
17. Kageji T, Nagahiro S, Horiguchi H, Watanabe T, Suzuya H, Okamoto Y. Successful high-dose chemotherapy for widespread neuroaxis dissemination of an opticohypothalamic juvenile pilocytic astrocytoma in an infant: A case report. J Neurooncol. 2003. 62: 281-7
18. Kano H, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for pilocytic astrocytomas part 1: Outcomes in adult patients. J Neurooncol. 2009. 95: 211-8
19. Kayama T, Tominaga T, Yoshimoto T. Management of pilocytic astrocytoma. Neurosurg Rev. 1996. 19: 217-20
20. Khalafallah AM, Jimenez AE, Shah PP, Brem H, Mukherjee D. Effect of radiation therapy on overall survival following subtotal resection of adult pilocytic astrocytoma. J Clin Neurosci. 2020. 81: 340-5
21. Krieger MD, Gonzalez-Gomez I, Levy ML, McComb JG. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997. 27: 1-11
22. Lee KJ, Marchan E, Peterson J, Harrell AC, Quinones-Hinojosa A, Brown PD. Management and survival of adult patients with pilocytic astrocytoma in the national cancer database. World Neurosurg. 2018. 112: e881-7
23. Lizarraga KJ, Gorgulho A, Lee SP, Rauscher G, Selch MT, DeSalles AA. Stereotactic radiation therapy for progressive residual pilocytic astrocytomas. J Neurooncol. 2012. 109: 129-35
24. Mair MJ, Wohrer A, Furtner J, Simonovska A, Kiesel B, Oberndorfer S. Clinical characteristics and prognostic factors of adult patients with pilocytic astrocytoma. J Neurooncol. 2020. 148: 187-98
25. Mamelak AN, Prados MD, Obana WG, Cogen PH, Edwards MS. Treatment options and prognosis for multicentric juvenile pilocytic astrocytoma. J Neurosurg. 1994. 81: 24-30
26. Mazloom A, Hodges JC, Teh BS, Chintagumpala M, Paulino AC. Outcome of patients with pilocytic astrocytoma and leptomeningeal dissemination. Int J Radiat Oncol Biol Phys. 2012. 84: 350-4
27. McAuley E, Brophy H, Hayden J, Pettorini B, Parks C, Avula S. The benefit of surveillance imaging for paediatric cerebellar pilocytic astrocytoma. Childs Nerv Syst. 2019. 35: 801-5
28. Narayan A, Jallo G, Huisman TA. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: Case report and review of the literature. Neuroradiol J. 2015. 28: 536-9
29. Nishio S, Morioka T, Takeshita I, Shono T, Inamura T, Fujiwara S. Chemotherapy for progressive pilocytic astrocytomas in the chiasmo-hypothalamic regions. Clin Neurol Neurosurg. 1995. 97: 300-6
30. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005. 64: 479-89
31. Ono T, Takahashi M, Hatakeyama J, Oda M, Sahm F, Nanjo H. Clinical significance of molecular diagnosis of pilocytic astrocytoma: A case report. NMC Case Rep J. 2019. 6: 95-9
32. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014. 16: iv1-63
33. Pathak P, Kumar A, Jha P, Purkait S, Faruq M, Suri A. Genetic alterations related to BRAF-FGFR genes and dysregulated MAPK/ERK/mTOR signaling in adult pilocytic astrocytoma. Brain Pathol. 2017. 27: 580-9
34. Roelz R, Reinacher P, Jabbarli R, Kraeutle R, Hippchen B, Egger K. Surgical ventricular entry is a key risk factor for leptomeningeal metastasis of high grade gliomas. Sci Rep. 2015. 5: 17758
35. Sie M, de Bont ES, Scherpen FJ, Hoving EW, den Dunnen WF. Tumour vasculature and angiogenic profile of paediatric pilocytic astrocytoma; is it much different from glioblastoma?. Neuropathol Appl Neurobiol. 2010. 36: 636-47
36. Soliman RK, Budai C, Mundada P, Aljohani B, Rushing EJ, Kollias SS. Suprasellar pilocytic astrocytoma in an adult with hemorrhage and leptomeningeal dissemination: Case report and review of literature. Radiol Case Rep. 2016. 11: 411-8
37. Stuer C, Vilz B, Majores M, Becker A, Schramm J, Simon M. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer. 2007. 110: 2799-808
38. Tabash MA. Characteristics, survival and incidence rates and trends of pilocytic astrocytoma in children in the United States; SEER-based analysis. J Neurol Sci. 2019. 400: 148-52
39. Tamura M, Zama A, Kurihara H, Fujimaki H, Imai H, Kano T. Management of recurrent pilocytic astrocytoma with leptomeningeal dissemination in childhood. Childs Nerv Syst. 1998. 14: 617-22
40. Theeler BJ, Ellezam B, Sadighi ZS, Mehta V, Tran MD, Adesina AM. Adult pilocytic astrocytomas: Clinical features and molecular analysis. Neuro Oncol. 2014. 16: 841-7
41. Tibbetts KM, Emnett RJ, Gao F, Perry A, Gutmann DH, Leonard JR. Histopathologic predictors of pilocytic astrocytoma event-free survival. Acta Neuropathol. 2009. 117: 657-65
42. Trifiletti DM, Peach MS, Xu Z, Kersh R, Showalter TN, Sheehan JP. Evaluation of outcomes after stereotactic radiosurgery for pilocytic astrocytoma. J Neurooncol. 2017. 134: 297-302
43. Trunin I, Golanov AV, Konovalov AN, Shishkina LV, Gorlachev GE, Gorelyshev SK. Stereotactic radiotherapy and radiosurgery in treatment of patients with deep-seated pilocytic astrocytomas. Zh Vopr Neirokhir Im N N Burdenko. 2012. 76: 64-78
44. Wasilewski A, Mohile N. Durable response to bevacizumab in adults with recurrent pilocytic astrocytoma. CNS Oncol. 2018. 7: CNS26