- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
- Department of Plastic Surgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
- Department of Neonatology Fukuoka Children’s Hospital, Fukuoka, Japan.
- Department of Pediatric Surgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan.
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Japan.
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka, Japan.
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_995_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuya Murakami1, Ai Kurogi1, Yoshihisa Kawakami2, Yushi Noguchi3, Makoto Hayashida4, Satoshi O. Suzuki5, Nobutaka Mukae6, Takafumi Shimogawa6, Koji Yoshimoto6, Takato Morioka7. Refractory CSF leakage following untethering surgery performed 10 months after birth for enlarging terminal myelocystocele associated with OEIS complex. 30-Dec-2021;12:628
How to cite this URL: Nobuya Murakami1, Ai Kurogi1, Yoshihisa Kawakami2, Yushi Noguchi3, Makoto Hayashida4, Satoshi O. Suzuki5, Nobutaka Mukae6, Takafumi Shimogawa6, Koji Yoshimoto6, Takato Morioka7. Refractory CSF leakage following untethering surgery performed 10 months after birth for enlarging terminal myelocystocele associated with OEIS complex. 30-Dec-2021;12:628. Available from: https://surgicalneurologyint.com/surgicalint-articles/11312/
Abstract
Background: Terminal myelocystocele (TMC) is an occult spinal dysraphism characterized by cystic dilatation of the terminal spinal cord in the shape of a trumpet (myelocystocele) filled with cerebrospinal fluid (CSF), which herniates into the extraspinal subcutaneous region. The extraspinal CSF-filled portion of the TMC, consisting of the myelocystocele and the surrounding subarachnoid space, may progressively enlarge, leading to neurological deterioration, and early untethering surgery is recommended.
Case Description: We report a case of a patient with TMC associated with OEIS complex consisting of omphalocele (O), exstrophy of the cloaca (E), imperforate anus (I), and spinal deformity (S). The untethering surgery for TMC had to be deferred until 10 months after birth because of the delayed healing of the giant omphalocele and the respiration instability due to hypoplastic thorax and increased intra-abdominal pressure. The TMC, predominantly the surrounding subarachnoid space, enlarged during the waiting period, resulting in the expansion of the caudal part of the dural sac. Although untethering surgery for the TMC was uneventfully performed with conventional duraplasty, postoperative CSF leakage occurred, and it took three surgical interventions to repair it. External CSF drainage, reduction of the size of the caudal part of the dural sac and use of gluteus muscle flaps and collagen matrix worked together for the CSF leakage.
Conclusion: Preoperative enlargement of the TMC, together with the surrounding subarachnoid space, can cause the refractory CSF leakage after untethering surgery because the expanded dural sac possibly increases its own tensile strength and impedes healing of the duraplasty. Early untethering surgery is recommended after recovery from the life-threatening conditions associated with OEIS complex.
Keywords: Collagen matrix, Duraplasty, Ependyma, Gluteus muscle flap, Hydrodynamic pressure, Subarachnoid space
INTRODUCTION
Terminal myelocystocele (TMC) is a rare closed spinal dysraphism characterized by cystic dilatation of the terminal part of the central canal, surrounded by a subarachnoid space that then herniates dorsally to an extraspinal region through a posterior spina bifida.[
We present a case of TMC associated with OEIS in which the untethering surgery was deferred until 10 months after birth because of the delayed healing of the giant omphalocele and the respiratory instability due to hypoplastic thorax and increased intra-abdominal pressure. The TMC massively enlarged during the waiting period, and CSF leakage occurred after the untethering surgery, which required three surgical interventions. The optimal timing of the untethering surgery for TMC and the mechanism and treatment of the CSF leakage are discussed.
CASE REPORT
A healthy woman had a natural pregnancy. Prenatal magnetic resonance images (MRIs) examination performed at 34+4 weeks of gestation revealed a lumbosacral mass filled with CSF and an abdominal wall defect [
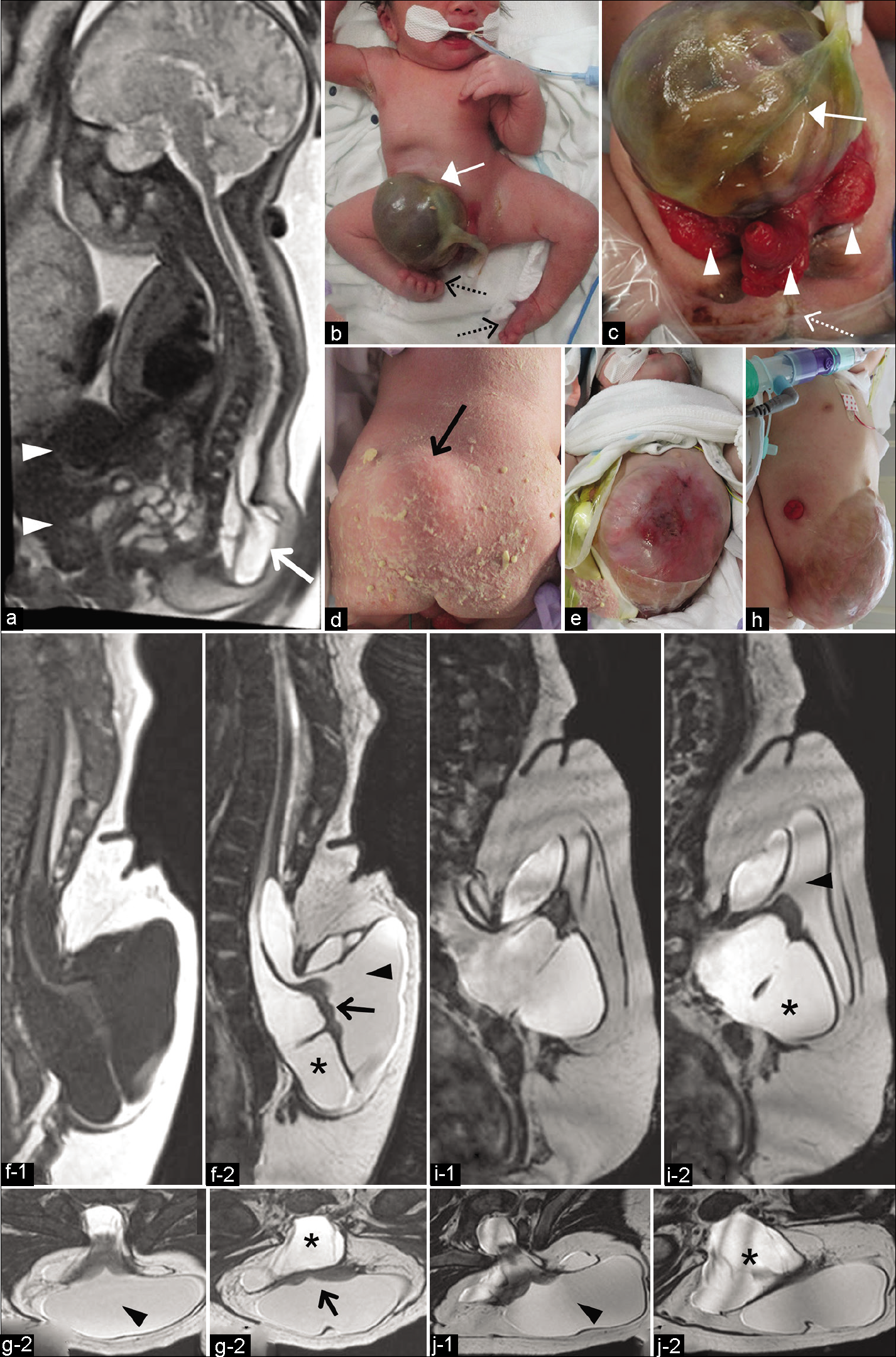
Figure 1:
(a) Prenatal half-Fourier acquisition single-shot turbo spin-echo sequence image at 34 weeks of gestation revealing skin-covered CSF-filled mass at the lumbosacral region (arrow) and an abdominal wall defect with omphalocele (arrowheads). (b-d) Photographs of the patient at birth showing omphalocele (white arrow), exstrophy of the cloaca (arrowheads), imperforate anus (dotted white arrow), mass at the lumbosacral region covered with normal skin (black arrow), and bilateral club foot (dotted black arrows). (e) Photograph of the patient at 5 months after birth showing partially inadequate epithelialization of the omphalocele surface. (f and g) MRI at 5 months after birth. 3D-T1-weighted (f-1) and 3D-heavily T2-weighted magnetic resonance imaging (3D-hT2WI) (f-2, g-1 and g-2) revealing a lowlying hydromyelic cord extruding into the extraspinal space with trumpet-shaped cystic cavity (myelocystocele; arrowhead) containing neural placode (arrow) and surrounded by large subarachnoid space (asterisk) extending posterocaudally into the extraspinal region. (h) Photograph of the patient at 9 months after birth showing the epithelialization of the protuberant omphalocele sac. Sagittal views (i) and axial views (j) of 3D-hT2WI at 9 months after birth (just before surgery). Note the more enlarged subarachnoid space (asterisk) compared to the myelocystocele (arrowhead). CSF: Cerebrospinal fluid, 3D-hT2WI: Three-dimensional heavily T2-weighted imaging.
MRIs at 5 months after birth, including three-dimensional heavily T2-weighted imaging (3D-hT2WI) and 3D-T1-weighted imaging (3D-T1WI),[
The surgery for the TMC was delayed until 10 months after birth, when sufficient stabilization of the respiratory status and epithelialization of the omphalocele sac would have been achieved, to ensure that the patient tolerated prone positioning during the operation. The abdomen remained protuberant, and definitive closure of the omphalocele was not performed at this time [
During surgery, the patient was placed prone with special management to protect his abdomen from pressure [
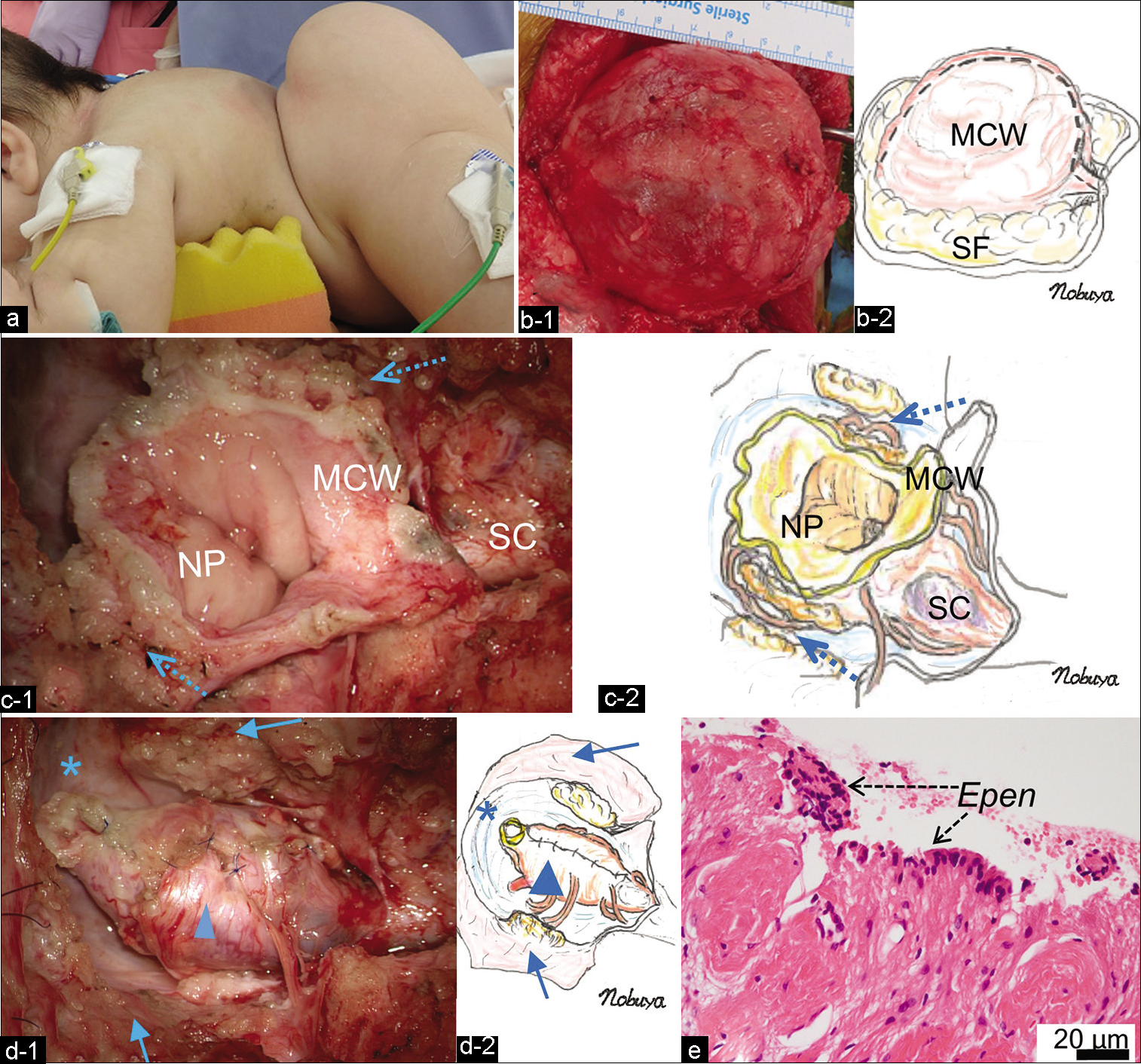
Figure 2:
(a) Photograph of the patient in the prone position during the operation. Note the extended lumbosacral mass. (b) Intraoperative photograph (b-1) and schematic drawing (b-2) after stripping the SF from the huge MCW. (c) Intraoperative photograph (c-1) and schematic drawing (c-2) demonstrating the untethered NP including part of the MCW, and the terminal portion of the hydromyelic SC. The untethering site is indicated by dotted arrows. (d) Intraoperative photograph (d-1) and schematic drawing (d-2) after pia-to-pia neurulation of the neural placode (arrowhead) demonstrating lipofibrous layers (arrows) composing a part of MCW and joining to the dural sac used for the subsequent duraplasty. Note the enlarged subarachnoid space in the caudal part of the dural sac (asterisk). (e) Histopathology of the inner wall of the myelocystocele sac demonstrating the ependymal layer (Epen) with the surrounding neuroglial tissues (hematoxylin and eosin staining). MCW: Myelocystocele sac wall, SF: Subcutaneous fat, NP: Neural placode, SC: Spinal cord.
Histopathological examination revealed that the inner wall of the myelocystocele sac was lined by an ependymal layer with surrounding glial fibrillary acidic protein-immunopositive neuroglial tissues, and it was histologically diagnosed as TMC [
Postoperatively, no de novo neurological abnormalities were observed. On the 2nd postoperative day (POD), however, CSF leakage occurred. Surgical intervention revealed a small fistula at the dural sac, and it was sutured. Although ventricular enlargement was not noted, an Ommaya CSF reservoir was placed at the right lateral ventricle to continuously drain the CSF and reduce the pressure in the lumbar subarachnoid space. The intracranial pressure estimated from the ventricular drain was within normal limit.
The patient was placed in a prone or a semi-decubitus position. However, high-pressure CSF leakage continued [
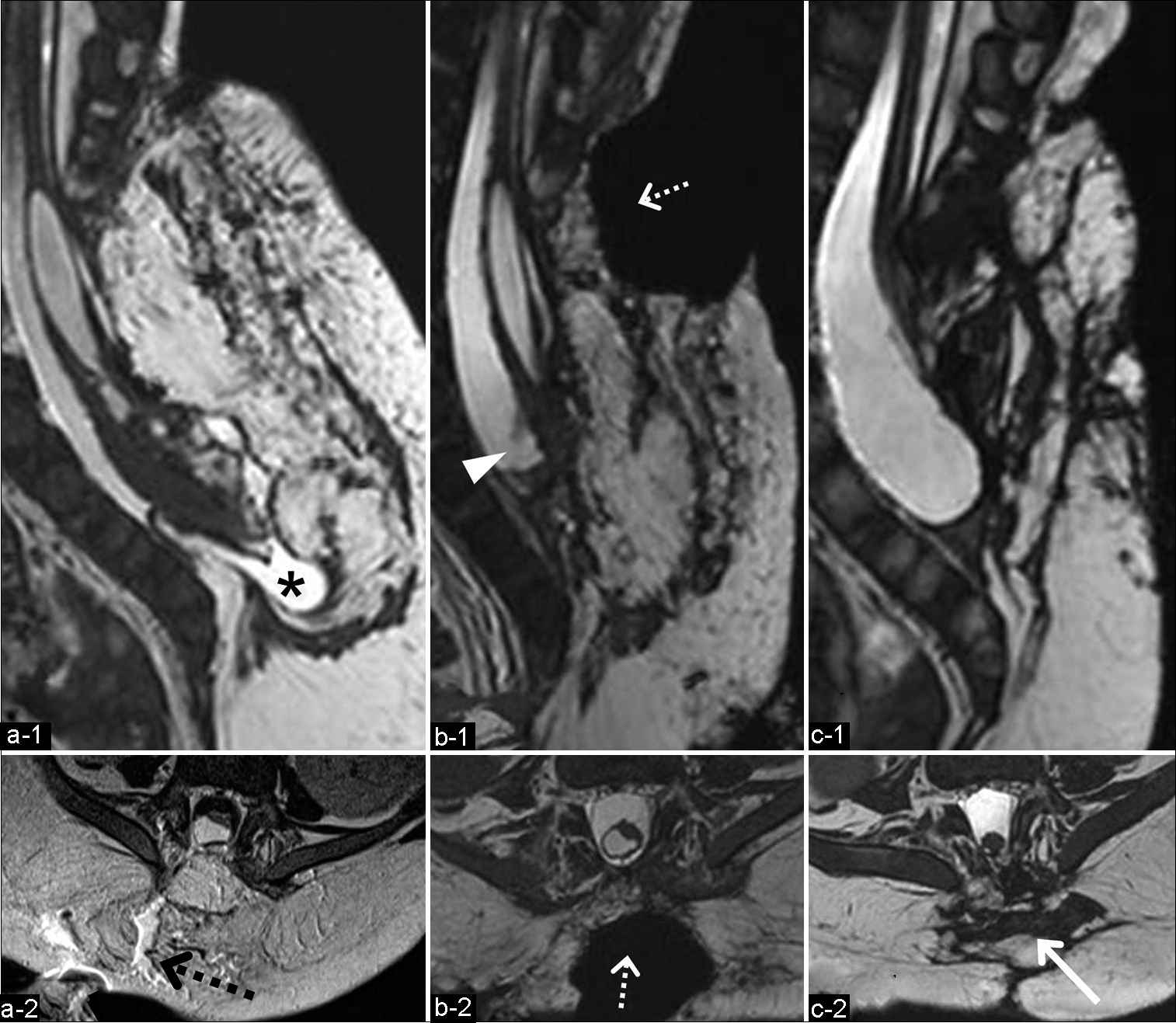
Figure 3:
(a) MRI after the first repair surgery for the CSF leakage showing the caudal subarachnoid space in the dural sac (asterisk) (a-1; 3D-hT2WI) and CSF leakage through the gaps between the subcutaneous tissue (black dotted arrow) (a-2; T2WI). Sagittal (b-1) and axial (b-2) views of 3D-hT2WI after the second repair surgery for CSF leakage revealing the deficit of the subcutaneous tissues and the dorsal aspect of the dural sac, which was covered with only thin tissue without muscle layers (white dotted arrow). Note the shortened terminal part of the reconstituted spinal cord and the dural sac (arrowhead). Sagittal (c-1) and axial (c-2) views of 3D-hT2WI 3 months after the third repair surgery (4 months after the untethering surgery) demonstrating the dorsal aspect of the dural sac covered with flaps of the gluteus muscles (arrow) without CSF leakage. CSF: Cerebrospinal fluid, 3D-hT2WI: Three-dimensional heavily T2-weighted imaging.
The third repair surgery on POD 20 revealed CSF oozing from the dural sac without any apparent fistula. A collagen matrix (DuraGen) was placed on the dural sac, and muscle turnover flaps employing the superficial layer of the gluteus maximus were used to cover the dural sac and reduce the dead space. Postoperatively, the CSF leakage decreased despite discontinuation of the external CSF drainage on POD 32 and eventually resolved. MRI 4 months after the untethering surgery revealed neither CSF leakage [
DISCUSSION
The pathoembryogenesis of TMC is considered to be a retained “terminal balloon” attached to the skin, which normally regresses, but can remain because of late arrest of the secondary neurulation before the degenerative phase.[
The extraspinal CSF-filled portion of TMC consists of the terminal cystic cavity of the spinal cord (myelocystocele) and the surrounding subarachnoid space. Hydrodynamic pressure and pulsation effect are assumed to cause enlargement of the extraspinal CSF-filled portion of closed spinal dysraphism.[
Corrective approaches for CSF leakage after untethering surgery include wound repair, fistula closure, and cystoperitoneal shunt.[
CONCLUSION
The present case demonstrates that early untethering surgery for TMC is recommended after recovery from the life-threatening conditions of OEIS complex, because the enlargement of the extraspinal subarachnoid space during the waiting period may cause postoperative CSF leakage as well as neurological deterioration.
Ethics statement
The authors confirm that written informed consent was obtained from the family of the infant described in this report.
The authors declare that this work complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research Foundation of Fukuoka Children Hospital.
Conflicts of interest
There are no conflicts of interest.
References
1. Choi S, McComb JG. Long-term outcome of terminal myelocystocele patients. Pediatr Neurosurg. 2000. 32: 86-91
2. Early CB, Fink LH. Some fundamental applications of the law of la place in neurosurgery. Surg Neurol. 1976. 3: 185-9
3. El-Hattab AW, Skorupski JC, Hsieh MH, Breman AM, Patel A, Cheung SW. OEIS complex associated with chromosome 1p36 deletion: A case report and review. Am J Med Genet A. 2010. 152A: 504-11
4. Gupta DK, Mahapatra AK. Terminal myelocystoceles: A series of 17 cases. J Neurosurg. 2005. 103: 344-52
5. Hashiguchi K, Morioka T, Samura K, Yoshida F, Miyagi Y, Nagata S. Holocord hydrosyringomyelia with terminal myelocystocele revealed by constructive interference in steady-state MR imaging. Pediatr Neurosurg. 2008. 44: 509-12
6. Jaiswal AK, Mahapatra AK. Terminal myelocystocele. J Clin Neurosci. 2005. 12: 249-52
7. Keppler-Noreuil KM. OEIS complex (omphaloceleexstrophyimperforate anus-spinal defects): A review of 14 cases. Am J Med Genet. 2001. 99: 271-9
8. Kim KH, Chong S, Lee JY, Kim K, Kim SK, Wang KC. Decreased MEPs during subcutaneous dissection for untethering surgery of a “true” lipomyelomeningocele: Aggravated traction of the spinal cord by release of the sac from the original nest. Childs Nerv Syst. 2019. 35: 529-33
9. Kim KH, Wang KC, Lee JY. Enlargement of extraspinal cysts in spinal dysraphism: A reason for early untethering. J Korean Neurosurg Soc. 2020a. 63: 342-45
10. Kim KH, Lee JY, Yang J, Park SH, Kim SK, Wang KC. Cystic retained medullary cord in an intraspinal J-shaped cul-de-sac: A lesion in the spectrum of regression failure during secondary neurulation. Childs Nerv Syst. 2020b. 37: 2051-6
11. Lee JY, Phi JH, Kim SK, Cho BK, Wang KC. Urgent surgery is needed when cyst enlarges in terminal myelocystoceles. Childs Nerv Syst. 2011. 27: 2149-53
12. Lee JY, Kim SP, Kim SW, Park SH, Choi JW, Phi JH. Pathoembryogenesis of terminal myelocystocele: Terminal balloon in secondary neurulation of the chick embryo. Childs Nerv Syst. 2013. 29: 1683-8
13. McLone DG, Naidich TP. Terminal myelocystocele. Neurosurgery. 1985. 16: 36-43
14. Morioka T, Hashiguchi K, Yoshida F, Nagata S, Miyagi Y, Mihara F. Dynamic morphological changes in lumbosacral lipoma during the first months of life revealed by constructive interference in steady-state (CISS) MR imaging. Childs Nerv Syst. 2007. 23: 415-20
15. Morioka T, Hashiguchi K, Yoshida F, Matsumoto K, Miyagi Y, Nagata S. Neurosurgical management of occult spinal dysraphism associated with OEIS complex. Childs Nerve Syst. 2008. 24: 723-9
16. Murakami N, Morioka T, Hashiguchi K, Yoshiura T, Hiwatashi A, Suzuki SO. Usefulness of three-dimensional T1-weighted spoiled gradient-recalled echo and three-dimensional heavily T2-weighted images in preoperative evaluation of spinal dysraphism. Childs Nerv Syst. 2013. 29: 1905-14
17. Narotam PK, Jose S, Nathoo N, Taylon C, Vora Y. Collagen matrix (DuraGen) in dural repair: Analysis of a new modified technique. Spine (Phila Pa 1976). 2004. 29: 2861-67
18. Pacilli M, Spitz L, Kiely EM, Curry J, Pierro A. Staged repair of giant omphalocele in the neonatal period. J Pediatr Surg. 2005. 40: 785-8
19. Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 1993. 32: 755-78
20. Pang D, Zovickian J, Lee YJ, Moes GS, Wang KC. Terminal Myelocystocele: Surgical observations and theory of Embryogenesis. Neurosurgery. 2012. 70: 1383-405
21. Pang D, Lee JY, Wang KC, Di Rocco C, Pang D, Rutka JT.editors. Secondary neurulation defects-2: Terminal myelocystocele: Surgical observations, laboratory findings, and theory of embryogenesis. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020. p.
22. Patel KB, Taghinia AH, Proctor MR, Warf BC, Greene AK. Extradural myelomeningocele reconstruction using local turnover fascial flaps and midline linear skin closure. J Plast Reconstr Aesthet Surg. 2012. 65: 1569-72
23. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90
24. Tokunaga S, Morioka T, Hashiguchi K, Samura K, Yoshida F, Miyagi Y. Double lumbosacral lipomas of the dorsal and filar types associated with OEIS complex: Case report. Neurol Med Chir (Tokyo). 2009. 49: 487-90
25. Udayakumaran S, Rathod CT. Tailored strategies to manage cerebrospinal fluid leaks or pseudomeningocele after surgery for tethered cord syndrome. World Neurosurg. 2018. 114: e1049-56
26. Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK. Neural differentiations of caudal cell mass (secondary neurulation) in chick embryos: Hamburger and Hamilton stages 16-45. Dev Brain Res. 2003. 142: 31-6
27. Yang HJ, Lee DH, Lee YJ, Chi JG, Lee JY, Phi JH. Secondary neurulation of human embryos: Morphological changes and the expression of neuronal antigens. Childs Nerv Syst. 2014. 30: 73-82
28. Zide BM, Epstein FJ, Wisoff J. Optimal wound closure after tethered cord correction. Technical note. J Neurosurg. 1991. 74: 673-6